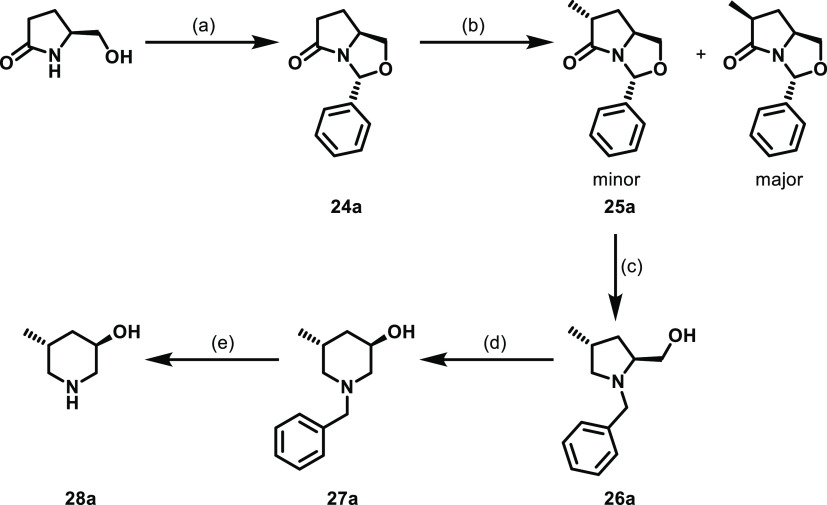
Scheme 2. Synthesis of trans-5-Methylpiperidin-3-ol (3R,5R) (28a).
Reagents and conditions: (a) benzaldehyde, p-TsOH, toluene, 120 °C, 16 h; (b) LDA, iodomethane, THF, −78 °C—rt, 4.5 h; (c) LiAlH4, THF, rt, 4 h; (d) TFAA, triethylamine, NaOH, THF, −78 °C to 65 °C, 16 h; and (e) Pd/C, H2, EtOH, rt, 3 h. The (3S,5S) isomer was made by a analogous scheme starting from R(d)-pyroglutaminol.