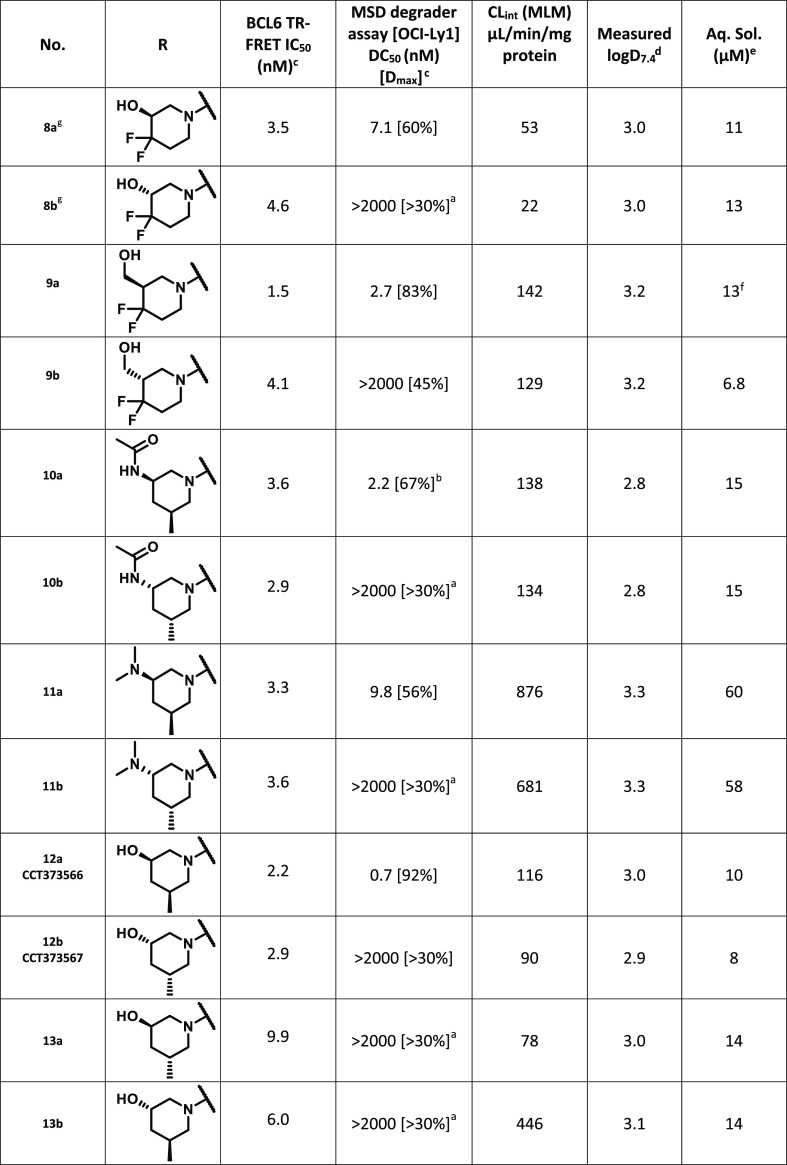
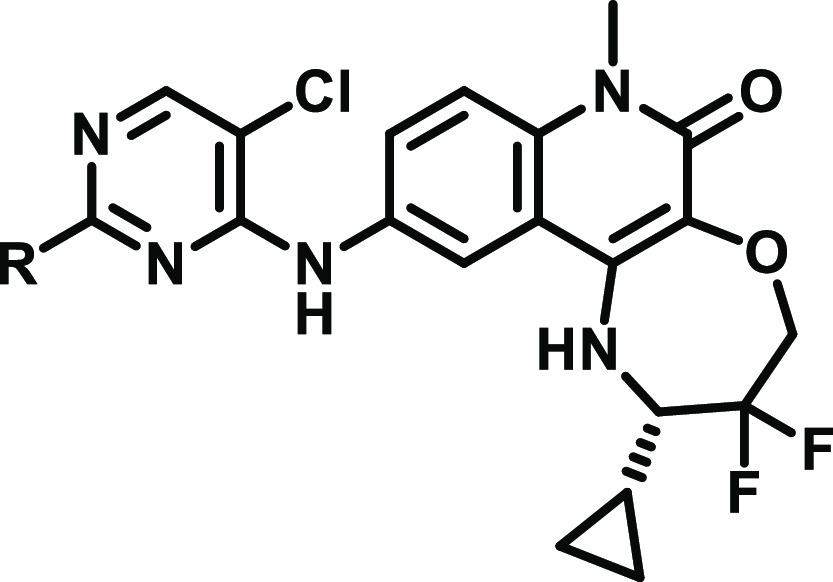
Table 2. Structure–Activity Relationships of Tricyclic Quinolinone Degraders.
Indicates n = 1.
Indicates n = 2.
Data represent the geometric mean of at least three replicates. See Tables S1 and S2 for full statistics.
Measured log D determined using the Chrom log D method.
Kinetic solubility measured by NMR in HEPES buffer at pH 8, containing 4% DMSO.
Indicates solubility measured by HPLC in PBS buffer and 1% DMSO at pH 7.4.
Compound exists as a single unknown enantiomer.