Figure 4.
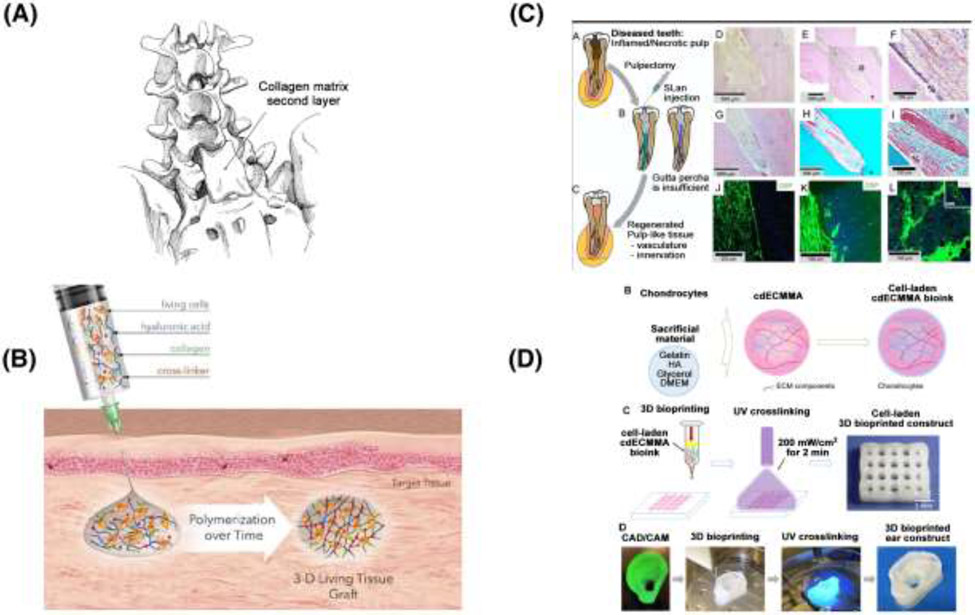
1st order hydrogels, which contain a CBER/CDER-regulated biologic, or a function-driven structure, but are manufactured using previously validated methods. (A) Depicts the surgical placement of a collagen patch for dura repair, an early example motivating the need for a current clinical trial for the use of Duragen seeded with ASCs for skull base surgery [155,156] (B) Depicts the mode of action of Biotime’s Renevia®, demonstrating how a living tissue graft is established in vivo. [160] (C) A schematic depicting supramolecular peptide-based hydrogels for dental pulp revascularization. [163] (D) Outlines the development of a decellularized ECM based bioink for auricular reconstruction, a variation of which is currently in clinical trials [167,168]