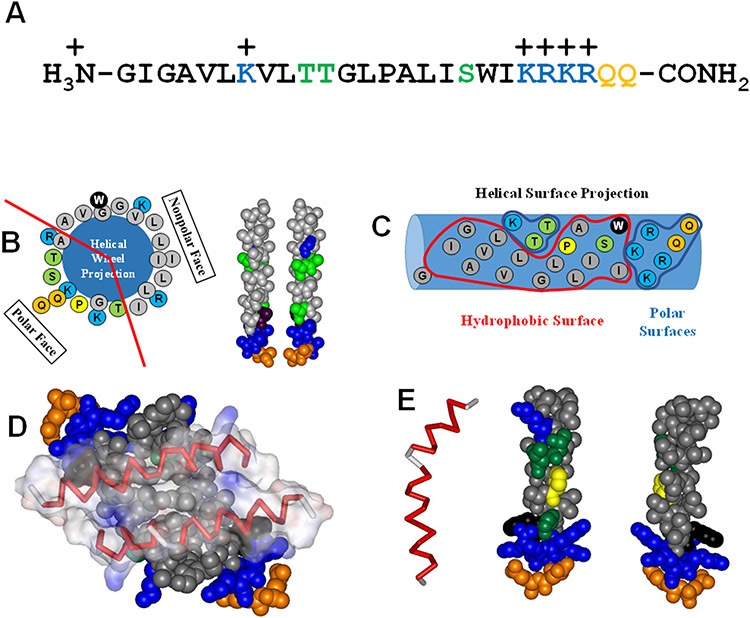
Figure 3.
Sequence and structure of melittin. Hydrophoboic residues are black. Moderately polar residues (S,T) are green. More polar glutamines (Q) are orange. Basic lysines (K) are blue. A. The amino acid sequence of melittin from A. melifera. B. The helical wheel diagram and idealized helical structures show that when melittin folds into an α-helix the structure is highly amphipathic, with hydrophobic and hydrophilic residues clustered on opposite faces. C. This helical surface projection shows that the amphipathicity of the melittin helix is two-dimensional, both on the face of the helix, and along the helix length where the C-terminal portion on the right is highly polar. D. In solution, the amphipathicity of melittin drives the formation of tetramers with the hydrophobic surfaces forming the interior of the structure. E. The Gly-Leu-Pro sequence at positions 12-14 break the melittin structure into two helical segments. This helix break is critical for the biological activity of melittin.