FIGURE 2.
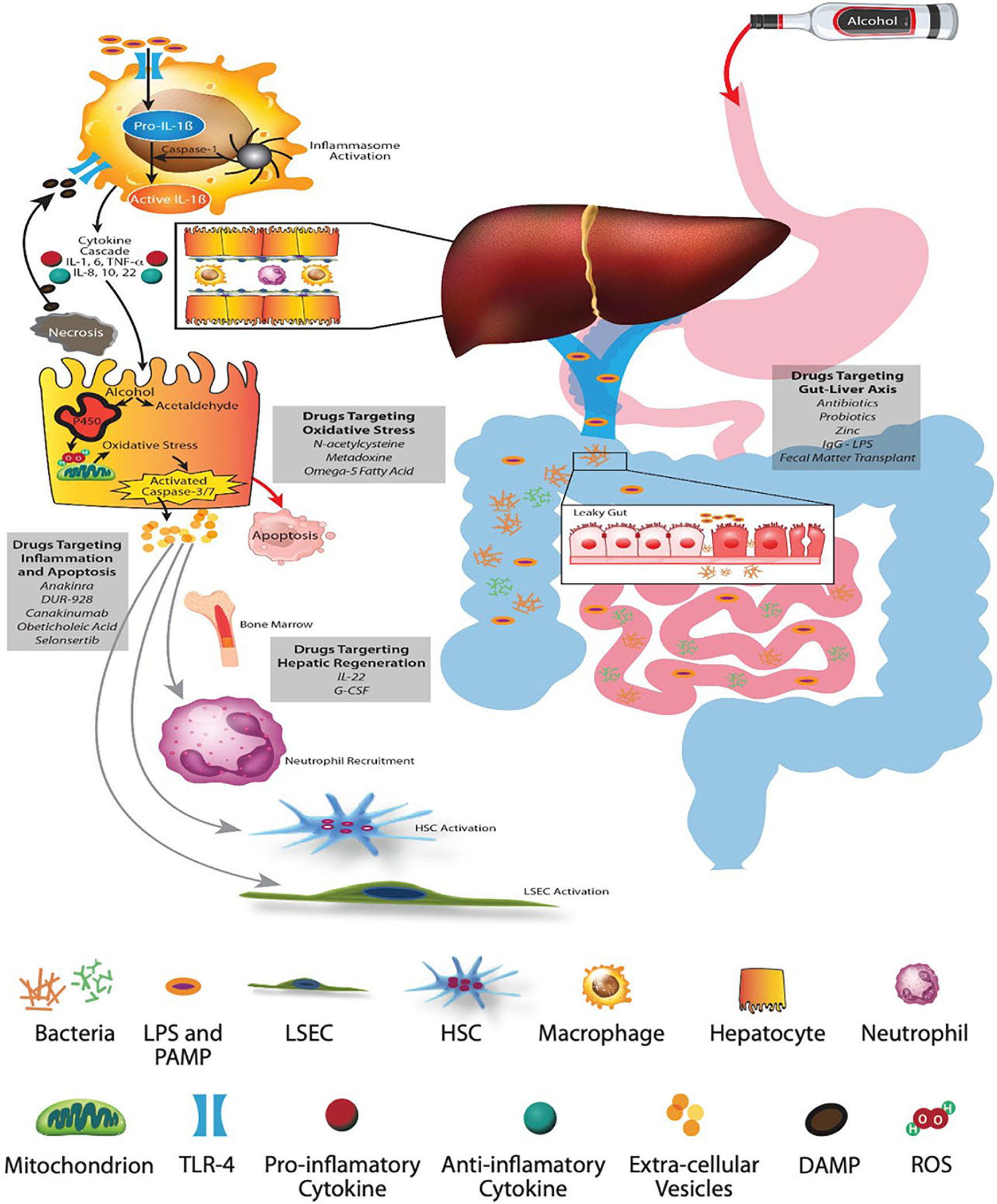
Pathophysiology of alcohol-associated liver disease and AH. (A) Alcohol-mediated increased gut permeability with leaky gut results in translocation of pathogen-associated molecular patterns and bacterial lipopolysaccharides (LPS) through the portal vein. (B) Schematic representation of the hepatic lobule with hepatocytes and sinusoids lined with liver sinusoid endothelial cells (LSECs) containing macrophages and neutrophils, and space of Disse containing HSCs. (C) LPS binds to membrane and cytosolic receptors of hepatocyte immune cells, macrophages, HSCs, and sinusoidal endothelial cells. LPS and its binding protein complex activates toll-like receptor-4 on the surface of hepatic macrophages, leading to an inflammatory cascade and signaling of chemokines and cytokines. The inflammasome complex (pro-IL-1β and caspase-1) activates pro-caspase-1 to generate IL-1β. (D) The metabolism of alcohol to acetaldehyde causes direct hepatocyte injury with generation of reactive oxygen species, leading to mitochondrial dysfunction, oxidative stress, and hepatocyte apoptosis. Damage associated molecular patterns released from the injured hepatocytes, especially HMGB-1 (high mobility group box protein 1) and microRNA-122, perpetuate ongoing hepatocyte injury. (E) Extracellular vesicles released from injured hepatocytes along with chemokines and cytokines recruit neutrophils from the bone marrow to the hepatic circulation and sinusoids. The inflammatory cascade cross talks with sinusoidal cells, resulting in activation of LSECs and HSCs, leading to portal hypertension and laying down of collagen with development of fibrosis
