Figure 4.
Properties of the CENP-LN vault
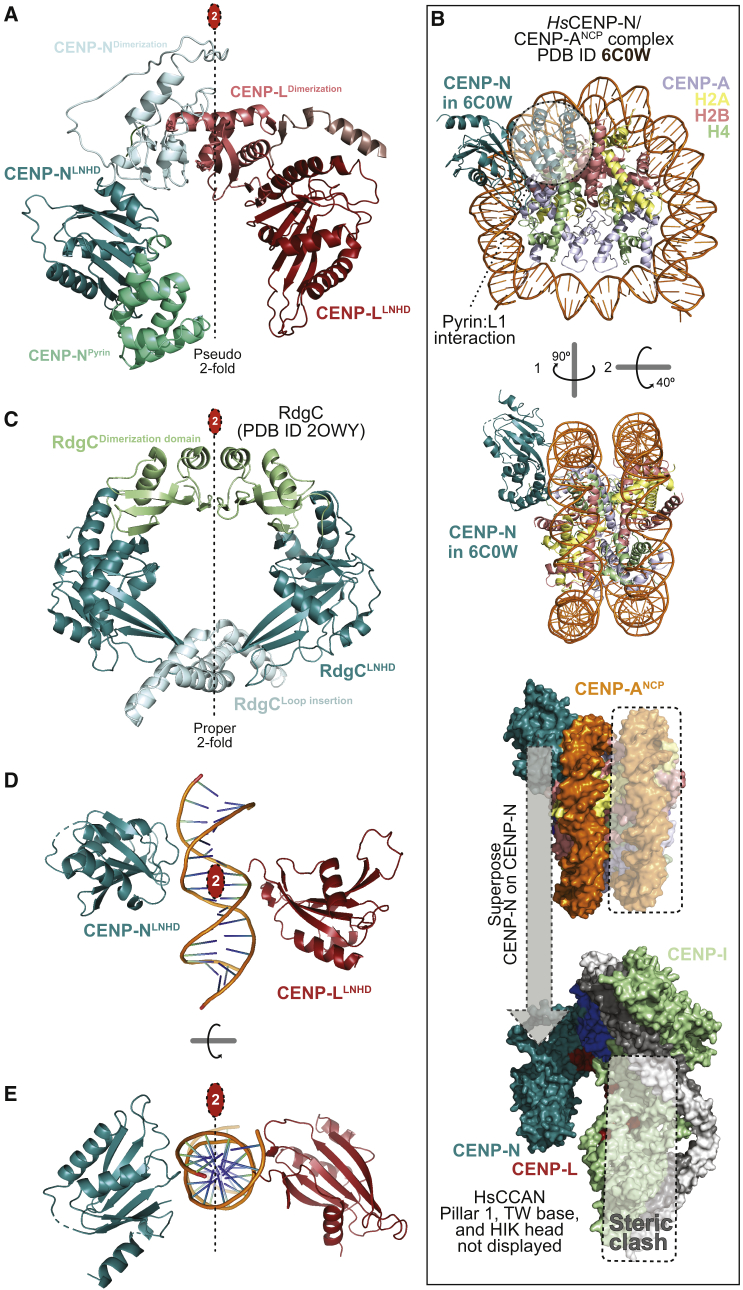
(A) The isolated CENP-LN vault with highlighted CENP-N pyrin domain (∼80 residues, greencyan), CENP-N LNHD (LN homology domain), dimerization domains of CENP-N and CENP-L, and CENP-L LNHD. A 2-fold pseudosymmetry axis (interrupted line with red oval) relates the LNHDs.
(B) Top: Rotated cartoon model of the CENP-N-terminal region bound to a CENP-A nucleosome (PDB: 6C0W) in two orthogonal orientations (Pentakota et al., 2017). The pyrin domain binds the L1 loop of CENP-A (light blue) and the LNHD to DNA. H2A C-terminal tails were removed for clarity. Bottom: Superposition of CENP-N in 6C0W to CENP-N in human CCAN predicts a steric clash of the second DNA gyre (boxed) onto pillar 2. The vault can only accept one gyre of DNA.
(C) Cartoon model of the RdgC homodimer highlighting the LNHDs and other structural elements.
(D) The LNHDs of CENP-LN displayed in absence of other domains (dimerization, pyrin) and modeled with 15-bp DNA from the 6C0W structure (B) positioned identically relative to CENP-N. The 2-fold pseudosymmetry axis between LNHDs coincides with the 2-fold pseudosymmetry axis of DNA.
(E) A 90°-rotated view of the same object.