Figure 1.
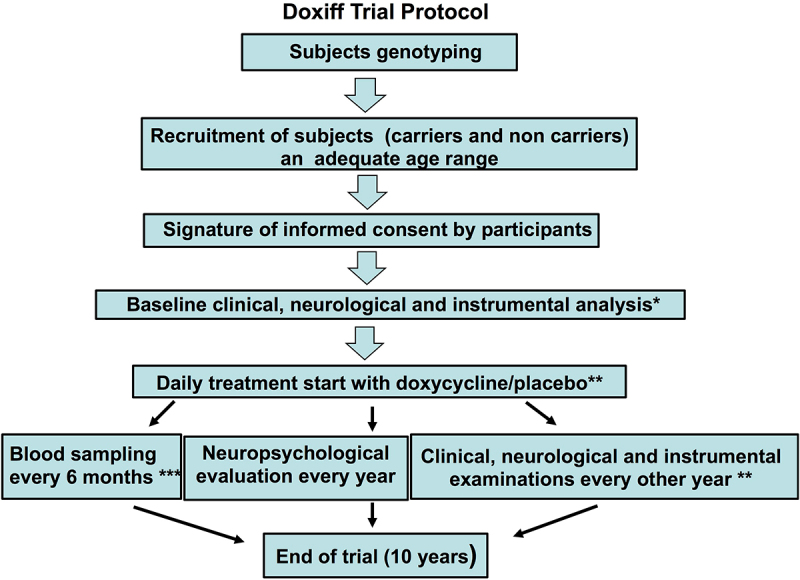
Flowchart of the clinical trial protocol.
*Selected subjects underwent of a full neurological examination including at baseline and at follow-up visits: (1) mental status and cognition (level of alertness, attention and cooperation, orientation, memory, language, calculations, right-left confusion, finger agnosia, agraphia, apraxia, neglect, sequencing tasks and frontal release signs, logic and abstraction, delusions and hallucinations, mood), (2) cranial nerves, (3) motor and sensory functions, (4) reflexes, (5) coordination and gait. The polysomnographic recording was done according to internationally accepted technical standards [54,55]. The specific variables monitored and recorded during polysomnographic assessment were global neural electroencephalographic activity, eye movements, submental electromyographic activity, heart rhythm, respiratory effort, nasal/oral airflow, oxygen saturation, body position and limb movements.** According to the approved amendment from May 2019 the doxycycline dosage was doubled, to two 100 mg tablets daily instead of one.*** The laboratory tests include fasting blood sugar, glycated haemoglobin, transaminase (SGOT and SGPT), direct and indirect bilirubin, gamma glutamyl transferase, alkaline phosphatase, albumin, prothrombin time, creatinine, BUN, full urine test, full cholesterol levels, HDL-cholesterol, triglyceride, complete blood count, protein electrophoresis, erythrocyte sedimentation rate and C-reactive protein. The plasma levels of cortisol, FT3 and FT4, free testosterone, 17 beta oestradiol, progesterone, 17 OH progesterone, DHEA-s, plasma renin activity and aldosterone and IGF-1 were investigated. The integrity of the hypothalamus-pituitary axis and correct response of the complex neuroendocrine network was determined by the response of the GH after GHRH+arginine test.
