Abstract
With this cohort we previously demonstrated preservation of thigh lean tissue with neuromuscular electrical stimulation combined with protein supplementation (NMES+PRO) treatment during bed rest in healthy older adults. Since macrophage polarization plays a significant role in the repair and maintenance of muscle size and insulin sensitivity, we hypothesized that muscle macrophages would be induced by NMES+PRO and would correspond to an increase in lean mass and an attenuated insulin resistance response altered by bed rest. Older adults (60–80y; BMI <30 kg/m2) underwent 5-days of bed rest and were randomized to either thrice daily treatment of NMES+PRO(n=8) or CON (n=8). Lean mass, insulin sensitivity, and markers of muscle macrophages, inflammation and connective tissue were determined PRE and POST bed rest. Glucose intolerance and insulin resistance occurred after bed rest but there was not a treatment effect (p>0.10). Pro-inflammatory-like macrophages (CD11b+,CD206-) increased (p<0.05) with NMES+PRO treatment and was different than CON. Minor changes in non-contractile tissue were observed. However, changes in muscle macrophages or extra-cellular matrix were not related to the preservation of thigh lean mass or insulin resistance. Daily NMES+PRO treatment during bed rest induced a muscle pro-inflammatory-like macrophage response and was unrelated to muscle size or metabolic function. Study listed as clinical trial NCT02566590.
Keywords: aging, fibrosis, glucose tolerance, immune cells, disuse, NMES
INTRODUCTION
Older adults demonstrate a greater prevalence of inactivity, symptoms of pre-diabetes and have reduced muscle mass and strength compared to younger adults (Oikawa et al. 2019). Because of these factors there is an increased risk of developing co-morbidities and reduced health span. Additionally, older adults are more frequently hospitalized with bed rest events that accelerate the potential development of co-morbidities through a rapid loss of muscle mass and onset of metabolic dysfunction (Bell et al. 2016; English and Paddon-Jones 2010; Oikawa et al. 2019). These negative events occur in less than a week (Kouw et al. 2019) and recovery can be impaired compared to younger adults (Reidy et al. 2018a).
In the skeletal muscle, immune cells can reside as a mononuclear population between the myofibers with paracrine effects on the muscle via secreted cytokines (Pillon et al. 2013). Macrophages are a subset of these cells that are dynamic in nature as they can be activated or “polarized” on a spectrum from inflammatory (M1-like) to that of anti-inflammatory (M2-like) state (Kosmac et al. 2018; Wynn and Vannella 2016). The content/ratio and polarization state of these cells may influence insulin sensitivity (Ikeda et al. 2013) and tissue repair and regrowth (Spiller and Koh 2017). In particular, these cells must coordinate in a timely manner to start repair (M1-like state) and then resolve and maintain (M2-like state) the tissue (Spiller and Koh 2017). Homeostasis is altered in the surrounding tissues when an imbalance in these processes occurs. (Spiller and Koh 2017).
Skeletal muscle macrophages are dynamic during periods of physical inactivity and muscle growth (Koh and Pizza 2009; Walton et al. 2019), regrowth from disuse atrophy (Reidy et al. 2019c), altered by aging (Cui et al. 2019; Reidy et al. 2019c) and that they have a positive relationship with metabolic function during physical inactivity (Reidy et al. 2019b). Skeletal muscle macrophages are also modified by NMES (Mackey and Kjaer 2017; Mackey et al. 2008; Mackey et al. 2011; Nosaka et al. 2011; Vanderthommen et al. 2015) and they have been shown to alter composition of the ECM in skeletal muscle (Mackey and Kjaer 2017; Mackey et al. 2011; Wynn and Vannella 2016). With aging, the content of M2 macrophages is increased, (Cui et al. 2019) and likely dysfunctional, (Reidy et al. 2019a) such that skeletal muscle fibrosis (increased ECM) occurs. Additionally, electrical stimulation via acupuncture has been shown to attenuate muscle atrophy in rodents with chronic kidney disease (Hu et al. 2015) or from denervation (Su et al. 2016) through the actions of macrophages.
We have recently demonstrated that muscle loss in healthy older adults following 5 days of continuous bed rest can be rescued with combined neuromuscular electrical stimulation and protein supplementation (NMES+PRO) (Reidy et al. 2017). Since macrophages have established roles in muscle growth and metabolism, we anticipated that the muscle size maintenance we observed in older adults may be related to changes in the muscle macrophage profile. Although we concluded that the maintained thigh lean mass was not functional in regards to our assessment of muscle strength, it has yet to be determined if the lean mass maintained in this context may be beneficial from a metabolic standpoint, (Wolfe 2006) and if macrophages correspond to metabolic function. Therefore, we hypothesized that NMES+PRO treatment during bed rest would modify the skeletal muscle macrophage content and that thigh lean mass and insulin resistance would correspond to skeletal muscle macrophage abundance.
MATERIALS AND METHODS
Participants.
Twenty healthy older adults were recruited from around the Salt Lake City area utilizing radio advertisement and word of mouth. Participants were excluded during screening if they had: uncontrolled hypertension, diabetes, HIV, hepatitis B and C, hyper/hypothyroidism, cardiovascular, kidney, respiratory, vascular and liver disease, history of DVT, neurological disorders, recent cancer treatment (within 1yr of enrolling), osteopenia, depression, and alcohol substance abuse. Enrolled participants were modestly physically active as defined as weekly walks, hikes, bike rides, and/or participation in <2 sessions of aerobic or resistive-type activities/week. All subjects read and signed the informed consent form. The parent study took place at the University of Utah (Nov 2014-May 2016) and was approved by the University of Utah Institutional Review Board, which agrees with the Declaration of Helsinki. This study is registered at www.clinicaltrials.org (NCT02566590) and the overall methodology, design, subject characteristics, body composition, and original primary outcomes from the parent clinical trial have been previously published (Reidy et al. 2017).
Experimental Design.
Enrolled participants were randomized (blocked and balanced by sex) into one of two groups: neuromuscular electrical stimulation in addition to protein supplementation (NMES+PRO) or Control (CON) and underwent a 5-day bed rest study. Tissue composition (via DXA) and muscle function (leg strength and power) were determined before and immediately after bed rest. Participants in the intervention group (NMES+PRO) received 40 minutes of NMES (3x/day) followed by protein supplementation. Prior nutritional and physical activity was assessed consistent with our previous 5-day bed rest studies (Reidy et al. 2017; Tanner et al. 2015).
5-Day Bed Rest.
Total caloric intake during bed rest was pre-determined by the research dietician using the Harris-Benedict equation adjusted for no physical activity. Additionally, participants consumed a diet that provided a daily protein amount of ~1.0g protein/kg/d protein intake (independent of protein supplementation). Participants were also provided water or non-caloric flavored beverages ad libitum throughout the 5-days of bed rest. All enrolled participants took part in a short-term (5d) supervised bed rest study at the University of Utah Center for Clinical and Translational Science (CCTS) using an identical protocol and safety guidelines as we have described previously (Reidy et al. 2017; Tanner et al. 2015). Lean mass (isolated thigh region) was assessed via DXA, consistent with our previous 5-day bed rest studies (Reidy et al. 2017; Tanner et al. 2015).
On the first day of bed rest, after an overnight fast, a muscle biopsy under 1% lidocaine was sampled from the vastus lateralis using a modified Bergström needle approach with manual suction (Evans et al. 1982). The biopsy was repeated after bed rest (day 5) under identical circumstances as the experiment on Day 1, except the muscle biopsy occurred on the opposite leg. Muscle tissue was immediately washed with saline and dissected of visible non-muscle tissue, flash-frozen in liquid nitrogen, and stored at −80°C for later analysis. For histochemical analysis, a portion of the baseline muscle biopsy was carefully mounted on cork in OCT then frozen in liquid nitrogen cooled in isopentane. Samples were placed in dry ice then transported to a −80°C freezer for later sectioning and immunofluorescence.
Daily Inpatient Intervention.
The NMES+PRO group underwent a two-pronged contraction and nutrition intervention that occurred 3 times a day (in the morning, afternoon, evening) on each day beginning on Day 1 and ending in the evening of Day 4 for a total of 12 sessions. However, two subjects at the initiation of the study received NMES+PRO two times a day during bed rest, but no difference was found between these two subjects and the remaining subjects in this group, so these subjects were pooled together.
The NMES protocol has been explained in detail elsewhere (Reidy et al. 2017). Briefly, self-adhesive surface electrodes, assisted by a trained nurse or investigator, were embedded on the sleeve and carefully placed on the vastus lateralis, vastus medialis and rectus femoris of both legs. The NMES protocol (EMPI Phoenix biphasic rectangular waveform stimulator; Clear Lake, SD) began with a 2min warm-up (6Hz, 300μs) in which the muscle twitched comfortably but did not contract. Immediately after, the protocol moved to the “work phase” for 20min (75Hz, 300μs). The work phase consisted of an intermittent contraction (4s) and rest period (10s). The work phase was followed by a 3-minute cool down (3Hz, 300μs). The NMES protocol was repeated two times in a row for a total work phase time per session of 44 minutes. To maintain the intensity level of the stimulus with each session, the participant was encouraged to manually increase the intensity during and with each subsequent session to the highest tolerable level. The subjects were also encouraged to physically contract on top of the NMES pulse.
Daily leucine-enriched whey protein supplementation (PRO) (BCAA Pepform; Glanbia Nutritionals, Twin Falls, ID) was provided 3 times a day to the NMES+PRO group. The protein beverage (80 kcal, 17g Protein, 1g Fat, 3g CHO) was made up of 12g essential amino acids (4.5g Leu) per serving. The protein supplement was mixed in water by CCTS staff and ingested by the participant within 1h following each NMES session. In order to account for extra calories consumed by the NMES+PRO group, the CON group also received a beverage 3 times a day that was similar in calories (80 kcal) to the protein supplement but absent of protein. CON group did not receive the NMES intervention.
Administration of an oral glucose tolerance test (OGTT) for N=10 for each group was conducted after a 10-h overnight fast occurred before bed rest and on the 4th day of bed rest. Measurements of insulin sensitivity included the HOMA-IR (Gutch et al. 2015), the Matsuda Index (Gutch et al. 2015) and the muscle insulin sensitivity index (Abdul-Ghani et al. 2007).
Serum glucose and insulin
Serum collected during the oral glucose tolerance test (OGTT) was assessed for glucose levels using a standard glucose analyzer (YSI, Yellow Springs, OH). Serum insulin levels (EMD Millipore, Billerica, MA) were determined at baseline (0 mins) and the final time point of the OGTT (120 mins) according to manufacturers’ instructions.
Immunofluorescence
Only N=8 was employed in each group due to tissue availability. Samples were removed from the cork at −25°C in a Microtome PLUS (Triangle Biomedical Sciences) where they were individually cut in 8 μm cross-sections. Muscle samples at PRE and POST for the same subject were placed on the same slide (Fisherbrand Superfrost®/Plus microscope slides; Fisher Scientific, USA). Several slides were generated per subject, for 1) macrophages with CD11b/CD206 co-staining, 2) T-cells with a CD3 staining and ECM characterization with wheat germ agglutinin (WGA) and 3) total collagen with sirius red staining. Following cutting, a hydrophobic marker (Vector, H-4000, Burlingame, CA) separated the sections, which were dried at room temperature (RT) and then stored at −20°C until analysis.
Skeletal muscle T-cell and WGA were conducted on the sample slide as follows. Sections were fixed in −20°C acetone at RT followed by 3×3 min rinses in PBS. Sections were incubated for 8 min with 3% H₂O₂ in PBS treatment at RT to block endogenous peroxidases. Following 3×3 min washes in PBS, sections were blocked for 60 min in 2.5% normal horse serum (NHS, Vector, S-2012) with vector avidin D solution (4 drops per 1 mL solution) at RT and subsequently incubated for 60 min at 4°C with primary antibody mouse anti-human CD3 (DAKO M7254, 1:100) diluted in 2.5% NHS with biotin solution block (4 drops per 1 mL solution). After 4×5 min rinses in PBS, slides were incubated for 30 min in secondary antibody donkey α mouse IgG biotin –SP-conjugated (Jackson Immuno, #715-065-150, 1:500) and orange-fluorescent tetramethylrhodamine WGA (Invitrogen, W7024, 1:50) diluted in 2.5% NHS. Sections were washed 4×5 min in PBS, and then incubated for 30 min in SA-HRP (Invitrogen, S-911, 1:500) diluted in PBS. After 3×5 min washes in PBS, sections were incubated for 10 min in Alexa flour 488 (1:500) in TSA kit solution (Invitrogen). Sections were washed 3×3 min in PBS, rinsed once with distilled water, and mounted with vector shield mounting media with DAPI (Vector, #H-1200). Slides were drained of excess mounting media, allowed to air dry at RT, and were subsequently stored at 4°C. Muscle T-cell content was quantified via density per unit area (range: 6–33 cells/mm2).
Skeletal muscle macrophage markers for CD11b/CD206 co-labeling were performed as previously described and have provided staining controls (Reidy et al. 2019b): Sections were fixed in −20°C acetone at RT followed by 3×3 min rinses in PBS. Sections were incubated for 8 min with 3% H₂O₂ in PBS treatment at RT to block endogenous peroxidases. Following 3×3 min washes in PBS, sections were blocked for 60 min in 2.5% normal horse serum (NHS, Vector, S-2012) with vector avidin D solution (4 drops per 1 mL solution) at RT and subsequently incubated for 60 min at 4°C with primary antibody mouse anti-human CD11b (Cell Sciences, #MON1019–1, 1:100) diluted in 2.5% NHS with biotin solution block (4 drops per 1 mL solution). After 4×5 min rinses in PBS, slides were incubated for 30 min in secondary antibody donkey α mouse IgG biotin –SP-conjugated (Jackson Immuno, #715-065-150, 1:500) diluted in 2.5% NHS. Sections were washed 4×5 min in PBS, and then incubated for 30 min in SA-HRP (Invitrogen, S-911, 1:500) diluted in PBS. After 3×5 min washes in PBS, sections were incubated for 10 min in Alexa flour 488 (1:500) in TSA kit solution (Invitrogen). Sections were washed 3×3 min in PBS, and blocked for 10 min in 2.5% NHS with vector avidin D solution at RT. Subsequent to a quick wash, the sections were incubated overnight in goat anti-human CD206 (R&D, #AF2534, 1:200) diluted in 2.5% NHS with biotin solution block at 4°C. On day 2 of staining, sections were washed 4×5 min, and then incubated for 60 min with a secondary antibody bovine anti-goat IgG (H+L) Cy3 (Jackson, #805-165-180, 1:250). Following 3×5 min rinses in PBS, sections were incubated for 30 min in primary antibody mouse IgG2a anti-human IgG Laminin (DSHB, 2E8, 1:250). After 3×5 min washes in PBS, the slides were incubated for 30 min in secondary antibody donkey-mouse IgG (H+L) AF647 (Jackson Immuno research, #715-605-151, 1:500). Sections were washed for 3×5 min in PBS, rinsed once with distilled water, and mounted with vector shield mounting media with DAPI (Vector, #H-1200). Slides were drained of excess mounting media, allowed to air dry at RT, and were subsequently stored at 4°C. This staining protocol for CD11b/CD206 resulted in DAPI positive nuclei (blue), laminin (purple- changed to white during image processing), CD11b (green) and CD206 (red). Validation of these stains was conducted previously (Reidy et al. 2019b). Immunofluorescent stained sections were imaged on a fully automated Nikon Ti-E inverted wide field microscope using a high sensitivity Hamastu camera at 200X magnification. Image processing and analysis was completed using Image J (FIJI). Muscle macrophage content was quantified via density per unit area (range: 3–127 cells/mm2). Macrophages were counted by determining overlap at CD11b+ DAPI+ (green-blue), CD206+ DAPI+ (green-red) andCD206+CD11b+DAPI+(green-red-blue) A recent protocol published confirms the validity of our chosen skeletal muscle macrophage protocols (Kosmac et al. 2018).
Sirius Red Staining and Analysis
Muscle sections were air dried for 1 h prior to fixation in Bouin’s solution (Labchem, Cat# LC117901) for 1 h at 56°C. After a series of washes in DI H¬2O, the samples were incubated in Picro Sirius Red solution (American MasterTech Scientific, Ref# STPSRPT) for 40 min. Samples were then rinsed in 0.5% glacial acetic acid followed by washes in DI H2O. Next, dehydration occurred via the following steps: incubation in 90% ethanol for 20 s, followed by 95% ethanol for 20 s then 100% ethanol for 20 s. The sections were then quickly dipped in xylene and mounted to glass cover slips using Cytoseal XYL (ThermoScientific, Ref# 8312–4).
Muscle Sirius Red staining was analyzed using Nikon Elements Advanced Research (Nikon, Japan) software. Briefly, thresholding of red-purple hues was defined to distinguish muscle fibers from collagen stained with Sirius Red. Regions of interest were demarcated around entire cross-sections and the percent area of Sirius Red was obtained.
mRNA Expression
mRNA expression of vastus lateralis muscle was conducted as follows (Reidy et al. 2018a). Total RNA was isolated by homogenizing 10–15 mg tissue with a hand-held homogenizer in a solution containing Tri reagent LS (Molecular Research Centre, Cincinnati, OH, USA). The RNA was separated and precipitated using chloroform and isopropanol. Extracted RNA was washed with ethanol then suspended in nuclease-free water with EDTA. RNA concentration was determined using the EPOCH (Take3; BioTek) spectrophotometer. cDNA was synthesized using a commercially available kit (iScript, BioRad, Hercules, CA, USA). All isolated RNA and cDNA samples were stored at −80°C until analysis. Real-time PCR was carried out with a CFX Connect real-time PCR cycler (BioRad) under similar protocol as reported previously (Drummond et al. 2014; Reidy PT 2016) using SYBR green custom designed primers for beta 2-microglobulin (β2M), collagen type I alpha 1 chain (Col1a), collagen type III alpha 1 chain (Col3a), Fibroblast growth factor 21 (FGF21), cluster of differentiation 68 (CD68), and C-C motif chemokine ligand 2 (CCL2) which have been previously described (Kwon et al. 2015). Cycle threshold values of target genes were normalized to β2M then fold change values were calculated (ΔΔCt). β2M remained stable across the interventions.
Statistical Analysis
Data were analyzed with an unpaired t-test (subject characteristics) and a 2-way ANOVA (BED REST, GROUP). Analysis was followed up with Tukey post hoc multiple comparison adjustment. Data are presented as mean ± SE. Analysis was carried out by GraphPad Prism version 8.0.
RESULTS
Subject Characteristics and Body Tissue Composition (Figure 1).
Figure 1.
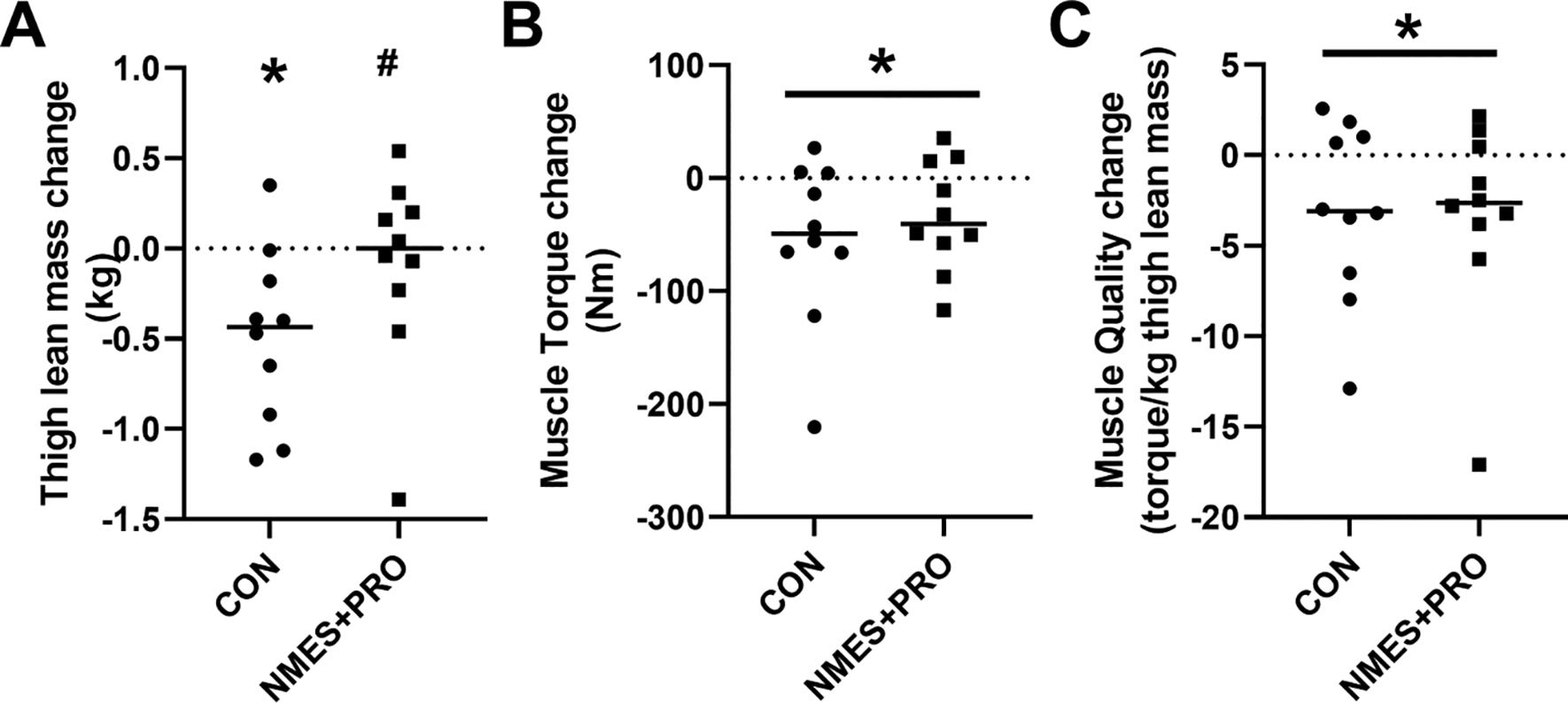
Thigh lean mass (A), isometric muscle torque (B) and muscle quality (C) lost during bed rest in healthy older adults following treatment with NMES+PRO or Control. NMES, Neuromuscular electrical stimulation; PRO, Protein. Individual data points and the bar is the Mean. # p<0.05 vs Control. * p<0.05 vs Pre.
There were no differences in the age or BMI for the Control (9M/1F: 69.4±2.1 y, 25.3±1.0 kg∙m2) or NMES+PRO groups (8M/2F: 68.9±2.1 y, 26.1±0.9 kg∙m2). The CON group lost (P<0.05) thigh lean mass after bed rest whereas the NMES+PRO group did not.
Glucose Tolerance and Insulin Sensitivity (Figure 2).
Figure 2.
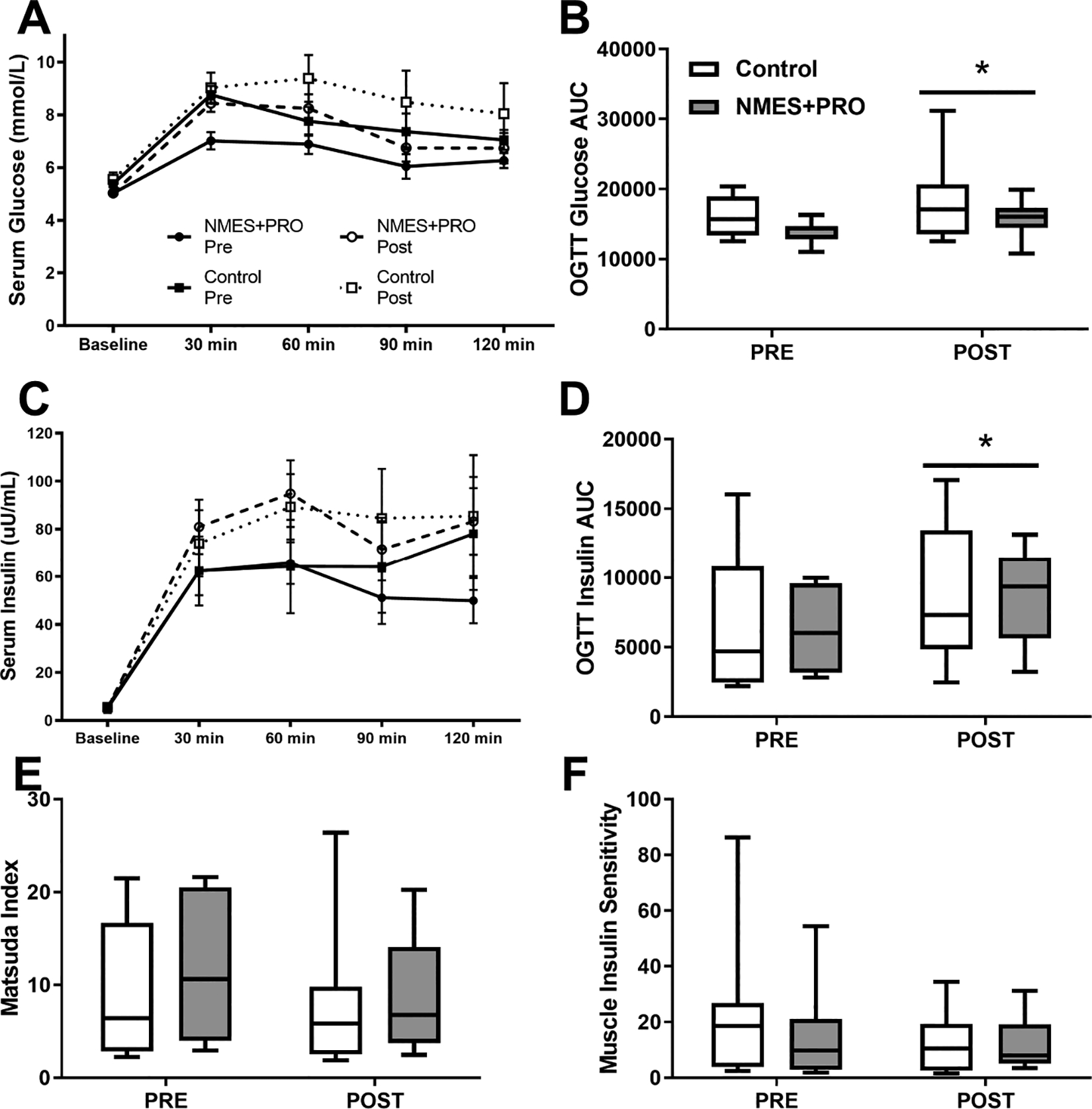
Assessment of glucose intolerance and indices of insulin sensitivity from an oral glucose tolerance test (OGTT) Pre and Post bed rest with NMES+PRO or Control treatment in healthy older adults. Glucose at OGTT timepoint (A) or area under the curve (AUC) (B). Insulin at OGTT timepoint (C) or area under the curve (AUC) (D). Matsuda (E) and Insulin Sensitivity (F) Index. NMES, Neuromuscular electrical stimulation; PRO, Protein. Data are Mean ± SEM. * p<0.05 vs Pre.
There was no change in baseline glucose or insulin following bed rest and no differences between groups (Figure 2). Serum glucose increased following bed rest (P>0.05) with no differences between groups during the OGTT (Fig 2A & B). Serum insulin increased following bed rest (P>0.05) with no differences between groups during the OGTT (Fig 2C & D). The Matsuda Index (Gutch et al. 2015) and Muscle Insulin Sensitivity (Abdul-Ghani et al. 2007) were not significantly altered by bed rest and were not different by group (Fig 2E & F).
Skeletal Muscle mRNA Expression (Table 1).
Table 1.
Skeletal Muscle mRNA Expression
| Pre | Post | ||
|---|---|---|---|
| Col1a | CON | 1.52±1.03 | 1.62±1.37 |
| NMES+PRO | 1.47±1.27 | 0.77±0.80 | |
| Col3a | CON | 1.24±0.97 | 0.83±0.56 |
| NMES+PRO | 1.39±1.43 | 1.26±1.07 | |
| FGF21 | CON | 1.40±1.19 | 0.95±0.95 |
| NMES+PRO | 1.38 ±1.43 | 1.17±1.01 | |
| CD68 | CON | 1.44±1.37 | 1.49±0.97 |
| NMES+PRO | 1.25±0.87 | 0.99±0.55 | |
| CCL2 | CON | 1.03±0.42 | 1.50±0.70# |
| NMES+PRO | 1.04±0.29 | 1.23±0.57# |
Data are Mean ± SD.
p<0.10 vs Pre.
There was no skeletal muscle mRNA expression (Col1a, Col3a, FGF21, CD68) changes after bed rest or as a result of treatment. However, there was a trend (p=0.086) for the gene encoding the chemokine Monocyte chemoattractant protein 1 (MCP-1), CCL2, to be increased following bed rest.
Skeletal Muscle Macrophages and T-cells.
CD11b+CD206− cells increased (p<0.05) following bed rest in the NMES+PRO group compared to baseline and compared to CON (Figure 3: CD11b+CD206−, CD11b−CD206+, CD11b+CD206+ cells). CD11b−CD206+, CD11b+CD206+ cells or the CD11b+CD206− to CD11b−CD206+ ratio were not altered following bed rest or different between groups. However, after visual inspection (Fig 3A & B), ~70–85% of the participants had lower CD11b−CD206+ cells following bed rest with a distribution of 6/10 in Control and a majority (7/8) in the NMES+PRO treatment. There were no changes in T-cells (Figure 4: CD3+), CD11b+CD206+ and CD11b−CD206+ cells with bed rest or as a result of treatment.
Figure 3.
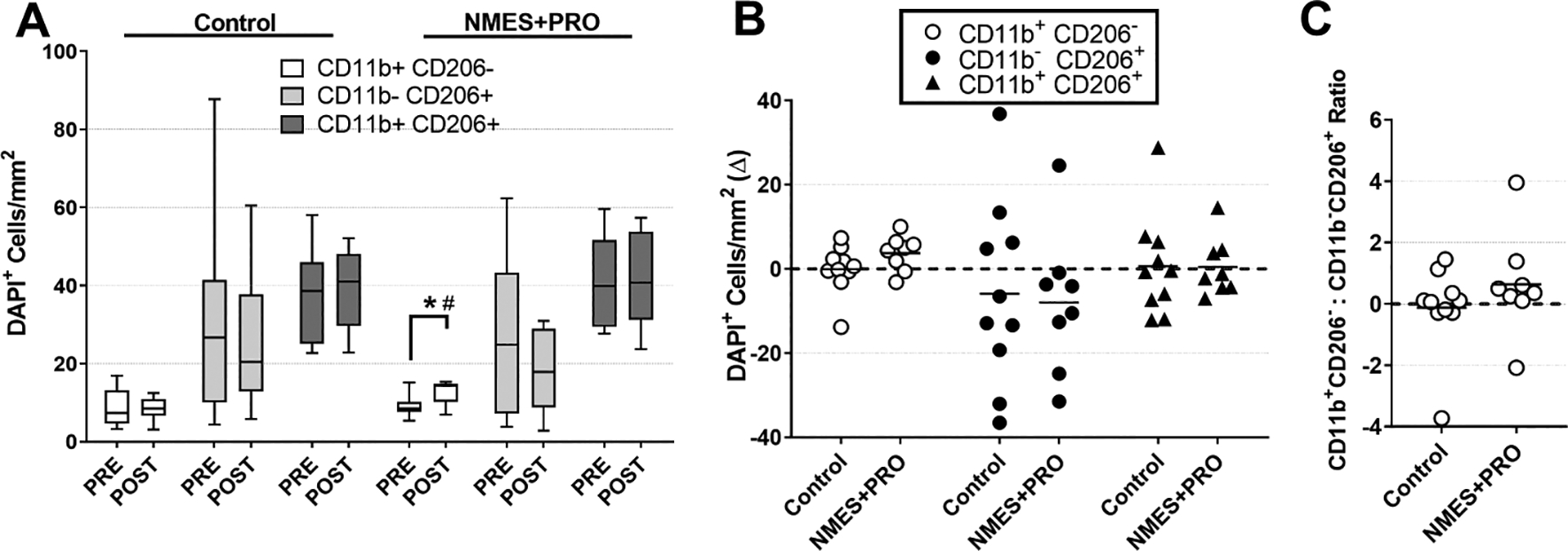
Immunofluorescent quantification of CD11b/CD206 macrophages on skeletal muscle cross sections Pre and Post bed rest with NMES+PRO or Control treatment in healthy older adults. NMES, Neuromuscular electrical stimulation; PRO, Protein. Data are Mean ± SEM as absolute values (A), Individual changes (B) with bar as the Mean change and (C) Change in the [CD11b+CD206−] to [CD11b−CD206+] ratio. # p<0.05 vs Control. * p<0.05 vs Pre.
Figure 4.
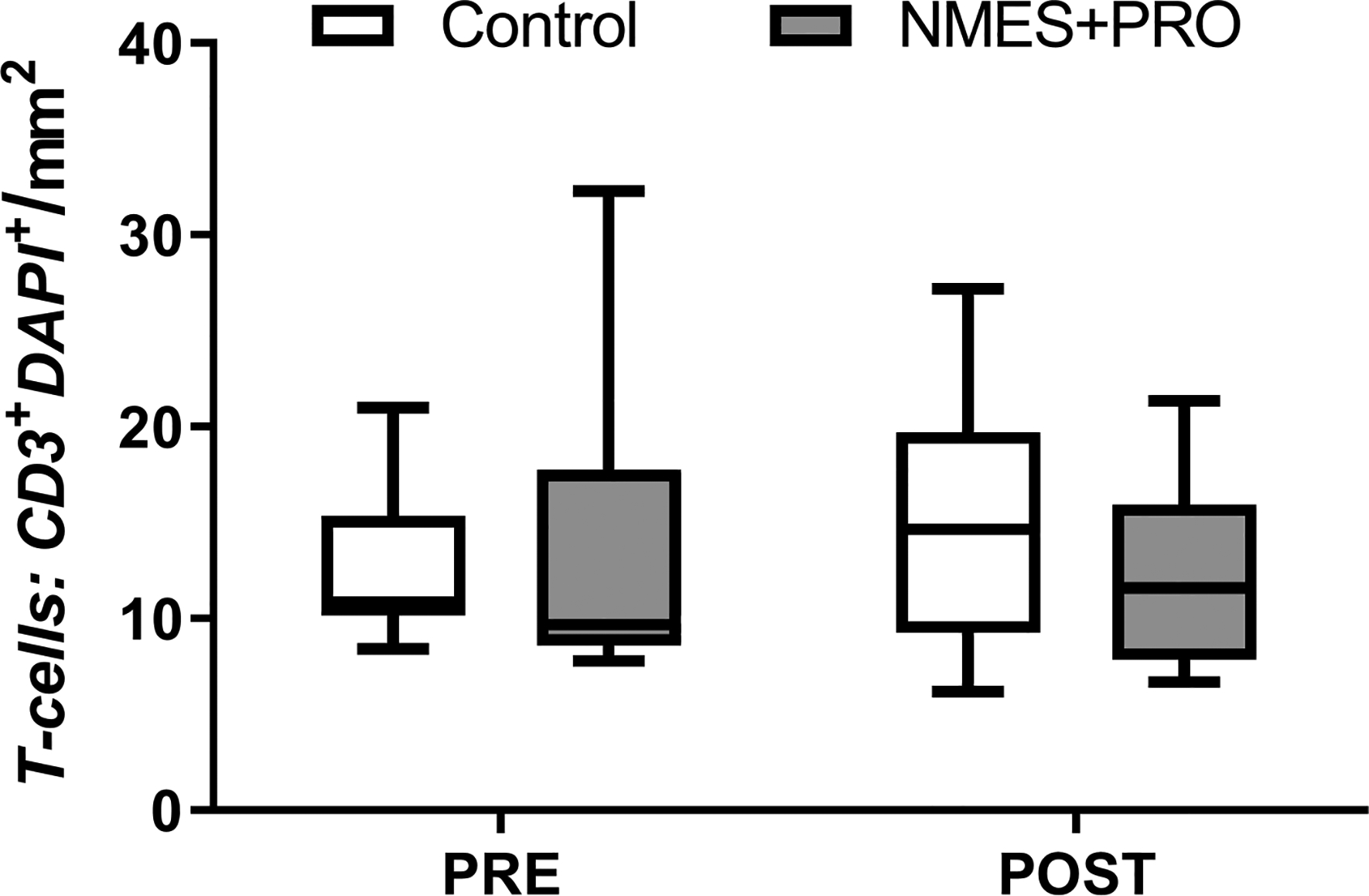
Immunofluorescent quantification of CD3 T-cells on skeletal muscle cross sections Pre and Post bed rest with NMES+PRO or Control treatment in healthy older adults. NMES, Neuromuscular electrical stimulation; PRO, Protein. Data are Mean ± SEM.
Skeletal Muscle Extracellular Matrix and Collagen (Figure 5).
Figure 5.
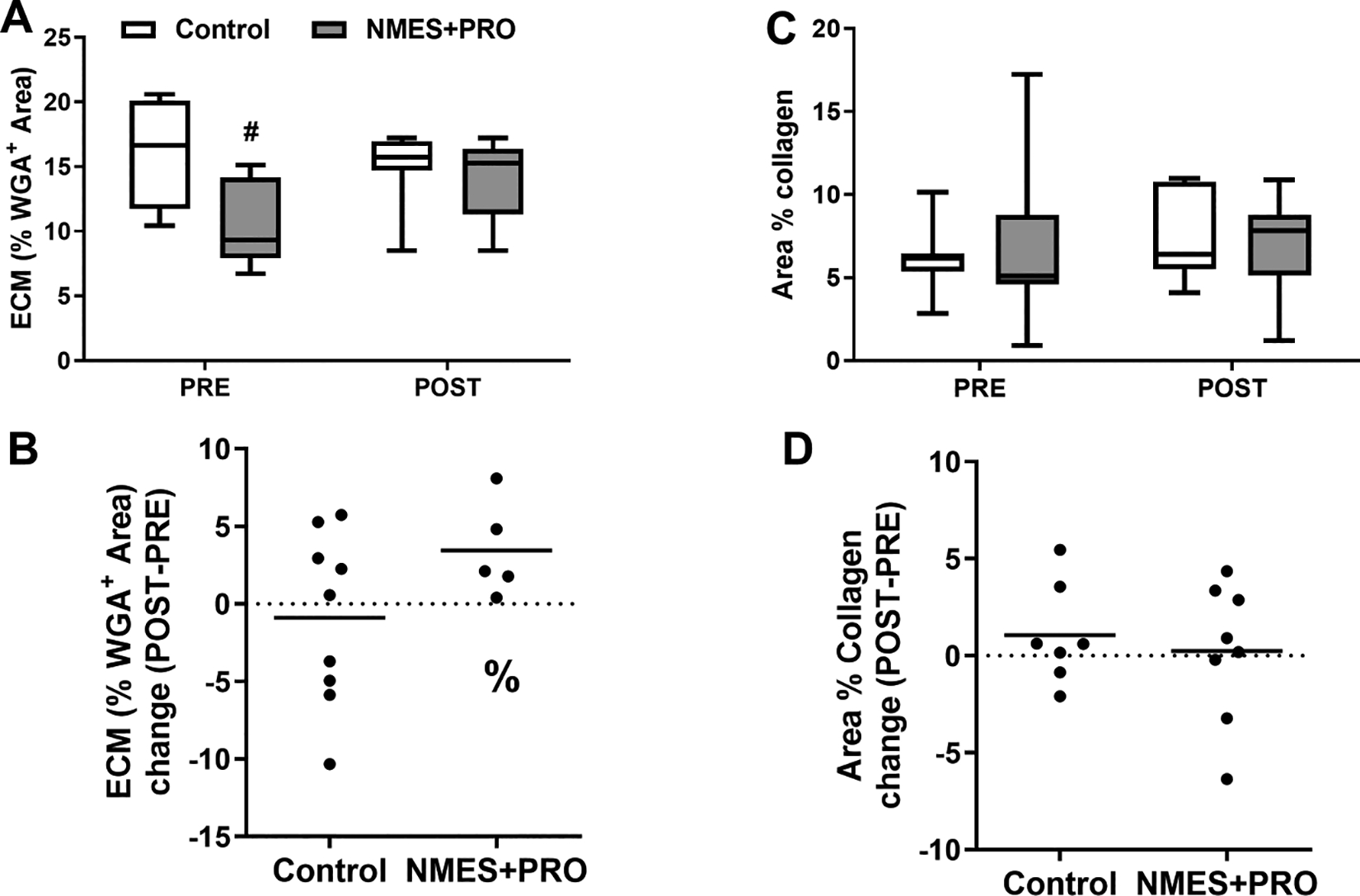
Immunofluorescent quantification of extra-cellular matrix (A,B) and collagen (C,D) on skeletal muscle cross sections Pre and Post bed rest with NMES+PRO or Control treatment in healthy older adults. Percent change from Pre is shown in panels B and D. ECM was quantitated using wheat germ agglutinin (WGA) and total collagen with sirius red staining. ECM, extra-cellular matrix; NMES, Neuromuscular electrical stimulation; PRO, Protein. Data are Mean ± SEM. # p<0.05 vs Control. % p<0.065 vs Pre.
Extracellular Matrix (ECM) was determined by wheat germ agglutinin (WGA) staining (Fig 5A & 5). % ECM was lower in the NMES+PRO group at baseline and tended (p=0.065) to increase increased after bed rest in the NMES+PRO group. There were no changes after bed rest or as a result of treatment in the skeletal muscle collagen content as determined by Sirius red staining (Fig 5 & D).
Correlations (Table 2).
Table 2.
Correlations between changes in skeletal muscle macrophages and changes in thigh lean mass, metabolic outcomes and non-contractile tissue after bed rest in healthy older adults following treatment with NMES+PRO or Control.
| CD11b+ CD206− | CD11b− CD206+ | CD11b+ CD206+ | CD11b+ CD206− | CD11b− CD206+ | CD11b+ CD206+ | |
|---|---|---|---|---|---|---|
| R-Values | P-Values | |||||
| Thigh Lean Mass | 0.372 | −0.439 | 0.091 | 0.141 | 0.102 | 0.728 |
| OGTT Glucose AUC | 0.008 | -0.551 | 0.159 | 0.977 | 0.033 | 0.541 |
| OGTT Insulin AUC | −0.180 | −0.152 | −0.069 | 0.490 | 0.589 | 0.791 |
| Matsuda Index | −0.004 | 0.275 | −0.114 | 0.987 | 0.320 | 0.662 |
| Muscle ISI | 0.087 | −0.176 | −0.212 | 0.748 | 0.548 | 0.432 |
| Collagen | 0.294 | −0.102 | 0.ss202 | 0.329 | 0.752 | 0.529 |
| ECM | 0.376 | 0.094 | 0.454 | 0.205 | 0.770 | 0.120 |
AUC, area under the curve; ECM, Extra-cellular matrix; ISI, insulin sensitivity index, OGTT, oral glucose tolerance test. Bolded values highlight a correlation (P<0.05).
There were no major correlations between changes (pre to post bed rest) in skeletal muscle macrophages and changes (pre to post bed rest) in thigh lean mass, metabolic outcomes and non-contractile tissue. However, we observed a moderate inverse correlation between the change in CD11b−CD206+ cells and the change in OGTT Glucose AUC (R=−0.551; p=0.033) and a trend for a similar effect with the Matsuda Index. (R=−0.682; p=0.063) that was nullified after removal of two outliers (R=−0.275; p=0.320).
DISCUSSION
We previously identified that daily neuromuscular electrical stimulation combined with protein ingestion prevented the loss of thigh lean mass in healthy older adults after 5 days of bed rest, yet the benefits of this therapeutic intervention did not extend to the loss of muscle strength (Reidy et al. 2017). Work from our laboratory (Reidy et al. 2019b; Reidy et al. 2019c) and others (Pillon et al. 2013; Reidy et al. 2019a; Saitou et al. 2018; Walton et al. 2019) has indicated a role of skeletal muscle macrophages with muscle size regulation and insulin sensitivity. Therefore, as a follow-up to the above study, we conducted a secondary analysis to determine if macrophages were related to changes in lean mass and insulin resistance in skeletal muscle. We found that daily treatment with NMES+PRO during bed rest induced an increase in the small population of skeletal muscle pro-inflammatory-like macrophages and increased connective tissue deposition, but these changes were largely unrelated to lean muscle mass and indices of muscle insulin sensitivity.
Our major finding was that NMES+PRO increased pro-inflammatory macrophages after bed rest and this was not related to changes in lean mass or indices of insulin resistance. Other laboratories have shown that NMES may modify skeletal muscle macrophages (Mackey and Kjaer 2017; Mackey et al. 2008; Mackey et al. 2011; Nosaka et al. 2011; Vanderthommen et al. 2015) while others do not (Gray et al. 2015; Kern et al. 2014). Discrepancies may be a result of a repeated bout effect, the type of contraction superimposed during NMES (isometric or eccentric) and/or the intensity of NMES stimulation. It appears that macrophages are increased in the days following an acute forced isometric (Mackey et al. 2008; Mackey et al. 2011) or eccentric contractions (Mackey and Kjaer 2017; Mackey et al. 2016) induced by NMES. However, 6-weeks of NMES training (5X a week for 30 min at 50 Hz) with COPD patients did not alter skeletal muscle macrophage content (CD163) (Gray et al. 2015), which was a similar finding as a 6-week NMES training study with healthy older adults (Kern et al. 2014). It is likely that most of the studies that show an increased macrophage abundance with NMES was due to NMES usage in an unaccustomed and high-volume manner to illicit muscle damage. It is also possible that the length of the intervention may be an important factor for detecting NMES-induced accumulation of macrophages. Most of the studies demonstrating increased macrophages were of a shorter duration. Since muscle disuse increases susceptibility to damage, especially with aging (Kanazawa et al. 2017), we cannot rule out that an increase in muscle macrophages with the NMES+PRO intervention was due to muscle damage.
One aspect of this hypothesis is that the intermittent reloading, mimicked to a degree, with NMES treatment during this period of bed rest may have caused muscle damage which triggered an accumulation of inflammatory macrophages. Myofiber degeneration occurs in both unloaded (Bigard et al. 1997; Brown and Hasser 1995; Haida et al. 1989; Krippendorf and Riley 1993) and reloaded myofibers (Bigard et al. 1997; Krippendorf and Riley 1993) while muscle disuse is well known for increasing the propensity of injury to exercise (Clarke et al. 1998; Kasper et al. 1990) and minimal contractile insults (Someya and Tachino 1997). Additionally, aging skeletal muscle is more susceptible to contraction-induced myofiber damage (Choi 2016). It appears that myofiber damage occurring during disuse and intermittent weight bearing is most prominent with weight bearing of shorter durations (Someya and Tachino 1997), where very long durations of inactivity are punctuated by very short periods of weight bearing – a phenomenon akin to this bed rest and NMES model, to a degree. Interestingly, as little of 2h of reloading followed by 22h of re-suspension in rats has been shown to elicit more damage than 24h of continuous reloading (Tidball and Wehling-Henricks 2007). As further support for our findings, macrophage accumulation was seen with disuse and intermittent weight bearing (Yamazaki T 1996). The increase in macrophages in our data set may be a positive adaptive response since intermittent muscle loading during disuse partially mitigated muscle atrophy (Brown and Hasser 1995; Herbert et al. 1988; Hurst and Fitts 2003; Miyazaki et al. 2008; Someya and Tachino 1997), which seems to be the case with reduced activity (Reidy et al. 2018b) as compared to the significant atrophy observed as a result of bed rest (Reidy et al. 2018a) with older adults.
CD11b+CD206−DAPI+ cells are classified as pro-inflammatory macrophages in human skeletal muscle (Kosmac et al. 2018) and represent a subset (~10–15%) of the macrophages found in skeletal muscle (Reidy et al. 2019b). Interestingly, these cells have been shown to decrease with human aging (Cui et al. 2019). The functional relevance of this smaller population of macrophages in skeletal muscle is not clear, especially with the sparse amount of human muscle macrophage data available combined with the lack of specificity regarding macrophage subpopulations. We hypothesize that this cell subpopulation may release a greater amount of inflammatory cytokines and reduce the development of fibrotic tissue (Spiller and Koh 2017), as is in contrast with aging which is characterized by an overabundance of M2 anti-inflammatory macrophages and fibrotic tissue (Wang et al. 2015; Wang et al. 2019). Though CCL2 (a macrophage chemoattractant) mRNA expression tended to increase with bed rest, this was not different between groups. However, we did not have sufficient tissue to thoroughly examine the skeletal muscle cytokine expression profile at the protein level so it remains to be determined if these M1-like cells promoted a greater inflammatory environment as a result of NMES+PRO. However, we did not observe an association between non-M1-like macrophages and ECM or collagen. This may be an indication of our low sample size or a lack of sensitivity of the measurement.
In addition to macrophages, we examined skeletal muscle T-cells since they may participate in tissue maintenance (Li et al. 2018). The total T-cells (CD3+ cells) were unaltered by bed rest or in response to either treatment. However, we were limited to CD3 detection alone (global detection of T-cells), therefore we cannot discount the possibility of a change in the subtype of T-cells present (e.g. helper or cytotoxic).
Finally, it is well established that aging skeletal muscle has a more fibrotic profile than younger muscle (Wang et al. 2015; Wang et al. 2019) and that muscle disuse promotes fibrosis (Seene et al. 2012). Moreover, macrophages partly regulate collagen synthesis. Therefore, we evaluated the levels of non-contractile tissue by IHC as total collagen and also immunofluorescent quantification of the extra-cellular matrix (ECM) with wheat germ agglutin (WGA) lectin. Though we found no change in total collagen after bed rest or in response to either treatment, surprisingly, we observed an increase in the WGA content with NMES+PRO after bed rest. However, this effect may be independent of the NMES+PRO treatment since the participants randomized to that group had lower ECM content at baseline coupled with that there were no changes in several transcripts for collagen and no associations between our assessment of the ECM content or thigh lean mass. Therefore, the relevance of this finding following NMES+PRO treatment remains to be determined. In conclusion, NMES+PRO treatment increased pro-inflammatory muscle macrophages and % ECM after bed rest but this response was largely independent of changes in lean mass and insulin resistance.
Novelty Bullets.
Neuromuscular electrical stimulation combined with protein supplementation (NMES+PRO) increased pro-inflammatory-like macrophages and ECM content in older adults after bed rest.
NMES+PRO changes in macrophages and non-contractile tissue macrophages did not correspond with muscle size preservation or insulin sensitivity.
ACKNOWLEDGEMENTS
We would like to thank the CCTS nursing, dietary and medical staff for their assistance with the muscle biopsies, blood sampling and patient care during the inpatient and outpatient visits. Finally, we appreciate the generosity of Glanbia Nutritionals for providing the pre-mixed EAA’s and Pepform BCCA supplements.
Funding was provided by the NIH R03AG047308, the University of Utah Funding Incentive Seed Grant Program awarded to M.J.D, National Institutes of Health under Ruth L. Kirschstein National Research Service Award NIH 1T32HL139451 from National Heart, Lung, and Blood Institute to Z.S.M., and the Summer Program for Undergraduate Research awarded to L.T.E. Clinical inpatient and outpatient services were supported by an institutional center grant (CTSA) provided by the National Center for Advancing Translational Sciences (UL1-TR001067).
Footnotes
Clinical Trials: NCT02566590
References
- Abdul-Ghani MA, Matsuda M, Balas B, and DeFronzo RA 2007. Muscle and Liver Insulin Resistance Indexes Derived From the Oral Glucose Tolerance Test. Diabetes Care 30(1): 89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- Bell KE, von Allmen MT, Devries MC, and Phillips SM 2016. Muscle Disuse as a Pivotal Problem in Sarcopenia-related Muscle Loss and Dysfunction. The Journal of frailty & aging 5(1): 33–41. doi: 10.14283/jfa.2016.78. [DOI] [PubMed] [Google Scholar]
- Bigard AX, Merino D, Lienhard F, Serrurier B, and Guezennec CY 1997. Quantitative assessment of degenerative changes in soleus muscle after hindlimb suspension and recovery. European journal of applied physiology and occupational physiology 75(5): 380–387. doi: 10.1007/s004210050176. [DOI] [PubMed] [Google Scholar]
- Brown M, and Hasser EM 1995. Weight-bearing effects on skeletal muscle during and after simulated bed rest. Arch Phys Med Rehabil 76(6): 541–546. Available from https://www.ncbi.nlm.nih.gov/pubmed/7763153 [accessed. [DOI] [PubMed] [Google Scholar]
- Choi S-J 2016. Age-related functional changes and susceptibility to eccentric contraction-induced damage in skeletal muscle cell. Integrative Medicine Research 5(3): 171–175. doi: 10.1016/j.imr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MSF, Bamman MM, and Feeback DL 1998. Bed rest decreases mechanically induced myofiber wounding and consequent wound-mediated FGF release. Journal of Applied Physiology 85(2): 593–600. doi: 10.1152/jappl.1998.85.2.593. [DOI] [PubMed] [Google Scholar]
- Cui CY, Driscoll RK, Piao Y, Chia CW, Gorospe M, and Ferrucci L 2019. Skewed macrophage polarization in aging skeletal muscle. Aging Cell 18(6): e13032. doi: 10.1111/acel.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC, et al. 2014. Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison. J Gerontol A Biol Sci Med Sci 69(8): 1040–1048. doi: 10.1093/gerona/glu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English KL, and Paddon-Jones D 2010. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13(1): 34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ, Phinney SD, and Young VR 1982. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14(1): 101–102. Available from http://www.ncbi.nlm.nih.gov/pubmed/7070249 [accessed. [PubMed] [Google Scholar]
- Gray A, Latimer L, Parmar A, Bradding P, Greening N, and Steiner M 2015. P18 The effects of acute and repeated bouts of unilateral Neuromuscular Electrical Stimulation on quadriceps muscle inflammation in COPD. Thorax 70(Suppl 3): A83–A84. doi: 10.1136/thoraxjnl-2015-207770.155. [DOI] [Google Scholar]
- Gutch M, Kumar S, Razi SM, Gupta KK, and Gupta A 2015. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab 19(1): 160–164. doi: 10.4103/2230-8210.146874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haida N, Fowler WM Jr., Abresch RT, Larson DB, Sharman RB, Taylor RG, et al. 1989. Effect of hind-limb suspension on young and adult skeletal muscle. I. Normal mice. Experimental neurology 103(1): 68–76. [DOI] [PubMed] [Google Scholar]
- Herbert ME, Roy RR, and Edgerton VR 1988. Influence of one-week hindlimb suspension and intermittent high load exercise on rat muscles. Experimental neurology 102(2): 190–198. Available from https://www.ncbi.nlm.nih.gov/pubmed/3181357 [accessed. [DOI] [PubMed] [Google Scholar]
- Hu L, Klein JD, Hassounah F, Cai H, Zhang C, Xu P, et al. 2015. Low-frequency electrical stimulation attenuates muscle atrophy in CKD--a potential treatment strategy. Journal of the American Society of Nephrology : JASN 26(3): 626–635. doi: 10.1681/asn.2014020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JE, and Fitts RH 2003. Hindlimb unloading-induced muscle atrophy and loss of function: protective effect of isometric exercise. J Appl Physiol (1985) 95(4): 1405–1417. doi: 10.1152/japplphysiol.00516.2002. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Tamura Y, Kakehi S, Takeno K, Kawaguchi M, Watanabe T, et al. 2013. Exercise-induced enhancement of insulin sensitivity is associated with accumulation of M2-polarized macrophages in mouse skeletal muscle. Biochem Biophys Res Commun 441(1): 36–41. doi: 10.1016/j.bbrc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Kanazawa Y, Ikegami K, Sujino M, Koinuma S, Nagano M, Oi Y, et al. 2017. Effects of aging on basement membrane of the soleus muscle during recovery following disuse atrophy in rats. Exp Gerontol 98: 153–161. doi: 10.1016/j.exger.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Kasper CE, White TP, and Maxwell LC 1990. Running during recovery from hindlimb suspension induces transient muscle injury. J Appl Physiol (1985) 68(2): 533–539. doi: 10.1152/jappl.1990.68.2.533. [DOI] [PubMed] [Google Scholar]
- Kern H, Barberi L, Lofler S, Sbardella S, Burggraf S, Fruhmann H, et al. 2014. Electrical stimulation counteracts muscle decline in seniors. Frontiers in aging neuroscience 6: 189. doi: 10.3389/fnagi.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TJ, and Pizza FX 2009. Do inflammatory cells influence skeletal muscle hypertrophy? Front Biosci (Elite Ed) 1: 60–71. [DOI] [PubMed] [Google Scholar]
- Kosmac K, Peck BD, Walton RG, Mula J, Kern PA, Bamman MM, et al. 2018. Immunohistochemical Identification of Human Skeletal Muscle Macrophages. Bio Protoc 8(12). doi: 10.21769/BioProtoc.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouw IWK, Groen BBL, Smeets JSJ, Kramer IF, van Kranenburg JMX, Nilwik R, et al. 2019. One Week of Hospitalization Following Elective Hip Surgery Induces Substantial Muscle Atrophy in Older Patients. J Am Med Dir Assoc 20(1): 35–42. doi: 10.1016/j.jamda.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Krippendorf BB, and Riley DA 1993. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle & nerve 16(1): 99–108. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- Kwon OS, Tanner RE, Barrows KM, Runtsch M, Symons JD, Jalili T, et al. 2015. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. American Journal of Physiology - Endocrinology and Metabolism 309(1): E11–E21. doi: 10.1152/ajpendo.00124.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tan J, Martino MM, and Lui KO 2018. Regulatory T-Cells: Potential Regulator of Tissue Repair and Regeneration. Frontiers in immunology 9: 585. doi: 10.3389/fimmu.2018.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, and Kjaer M 2017. The breaking and making of healthy adult human skeletal muscle in vivo. Skeletal Muscle 7(1): 24. doi: 10.1186/s13395-017-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Bojsen-Moller J, Qvortrup K, Langberg H, Suetta C, Kalliokoski KK, et al. 2008. Evidence of skeletal muscle damage following electrically stimulated isometric muscle contractions in humans. J Appl Physiol (1985) 105(5): 1620–1627. doi: 10.1152/japplphysiol.90952.2008. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Rasmussen LK, Kadi F, Schjerling P, Helmark IC, Ponsot E, et al. 2016. Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti-inflammatory medication. FASEB journal 30(6): 2266–2281. doi: 10.1096/fj.201500198R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Brandstetter S, Schjerling P, Bojsen-Moller J, Qvortrup K, Pedersen MM, et al. 2011. Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle. FASEB journal 25(6): 1943–1959. doi: 10.1096/fj.10-176487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Noguchi M, and Takemasa T 2008. Intermittent Reloading Attenuates Muscle Atrophy through Modulating Akt/mTOR Pathway. Medicine & Science in Sports & Exercise 40(5): 848–855. doi: 10.1249/MSS.0b013e318163275f. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Aldayel A, Jubeau M, and Chen TC 2011. Muscle damage induced by electrical stimulation. Eur J Appl Physiol 111(10): 2427–2437. doi: 10.1007/s00421-011-2086-x. [DOI] [PubMed] [Google Scholar]
- Oikawa SY, Holloway TM, and Phillips SM 2019. The Impact of Step Reduction on Muscle Health in Aging: Protein and Exercise as Countermeasures. Frontiers in Nutrition 6(75). doi: 10.3389/fnut.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon NJ, Bilan PJ, Fink LN, and Klip A 2013. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab 304(5): E453–465. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- Reidy PT, Dupont-Versteegden EE, and Drummond MJ 2019a. Macrophage Regulation of Muscle Regrowth From Disuse in Aging. Exerc Sport Sci Rev 47(4): 246–250. doi: 10.1249/jes.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy PT, McKenzie AI, Brunker P, Nelson DS, Barrows KM, Supiano M, et al. 2017. Neuromuscular Electrical Stimulation Combined with Protein Ingestion Preserves Thigh Muscle Mass But Not Muscle Function in Healthy Older Adults During 5 Days of Bed Rest. Rejuvenation Res 20(6): 449–461. doi: 10.1089/rej.2017.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy PT, Lindsay CC, McKenzie AI, Fry CS, Supiano MA, Marcus RL, et al. 2018a. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp Gerontol 107: 37–49. doi: 10.1016/j.exger.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy PT, Yonemura NM, Madsen JH, McKenzie AI, Mahmassani ZS, Rondina MT, et al. 2019b. An accumulation of muscle macrophages is accompanied by altered insulin sensitivity after reduced activity and recovery. Acta Physiol (Oxf) 226(2): e13251. doi: 10.1111/apha.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy PT, McKenzie AI, Mahmassani Z, Morrow VR, Yonemura N, Hopkins PN, et al. 2018b. Skeletal muscle ceramides and relationship to insulin sensitivity after two weeks of simulated sedentary behavior and recovery in healthy older adults. J Physiol. 596(21): 5217–5236. doi: 10.1113/JP276798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy PT, McKenzie AI, Mahmassani ZS, Petrocelli JJ, Nelson DB, Lindsay CC, et al. 2019c. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. American Journal of Physiology-Endocrinology and Metabolism 317(1): E85–E98. doi: 10.1152/ajpendo.00422.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou K, Tokunaga M, Yoshino D, Sakitani N, Maekawa T, Ryu Y, et al. 2018. Local cyclical compression modulates macrophage function in situ and alleviates immobilization-induced muscle atrophy. Clin Sci (Lond) 132(19): 2147–2161. doi: 10.1042/cs20180432. [DOI] [PubMed] [Google Scholar]
- Seene T, Kaasik P, and Riso EM 2012. Review on aging, unloading and reloading: changes in skeletal muscle quantity and quality. Archives of gerontology and geriatrics 54(2): 374–380. doi: 10.1016/j.archger.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Someya F, and Tachino K 1997. Effects of Various Daily Weight-Bearing Periods on Rat Soleus Muscle during Hindlimb Suspension Histochemical and Mechanical Properties. The Japanese Journal of Rehabilitation Medicine 34(6): 410–417. doi: 10.2490/jjrm1963.34.410. [DOI] [Google Scholar]
- Spiller KL, and Koh TJ 2017. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev 122: 74–83. doi: 10.1016/j.addr.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Hu L, Cheng J, Klein JD, Hassounah F, Cai H, et al. 2016. Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates denervation-induced muscle atrophy. Journal of Applied Physiology 120(4): 426–436. doi: 10.1152/japplphysiol.00175.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, et al. 2015. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 593(18): 4259–4273. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, and Wehling-Henricks M 2007. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol 578(Pt 1): 327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderthommen M, Chamayou R, Demoulin C, Crielaard JM, and Croisier JL 2015. Protection against muscle damage induced by electrical stimulation: efficiency of a preconditioning programme. Clin Physiol Funct Imaging 35(4): 267–274. doi: 10.1111/cpf.12160. [DOI] [PubMed] [Google Scholar]
- Walton RG, Kosmac K, Mula J, Fry CS, Peck BD, Groshong JS, et al. 2019. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci Rep 9(1): 969. doi: 10.1038/s41598-018-37187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wehling-Henricks M, Samengo G, and Tidball JG 2015. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 14(4): 678–688. doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wehling-Henricks M, Welc SS, Fisher AL, Zuo Q, and Tidball JG 2019. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB journal. 33(1): 1415–1427. doi: 10.1096/fj.201800973R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR 2006. The underappreciated role of muscle in health and disease. The American journal of clinical nutrition 84(3): 475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- Wynn TA, and Vannella KM 2016. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44(3): 450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T HN, Katsuhiko T 1996. Effect of weight-bearing frequency per day in retarding disuse atrophy in rat soleus muscle. Rigaku ryoho janaru 30(1): 53–57. [Google Scholar]


