Abstract
Objective Anterior skull base meningiomas include olfactory groove, planum sphenoidale, and tuberculum sellae lesions. Traditionally, standard craniotomy approaches have been used to access meningiomas in these locations. More recently, minimally invasive techniques including supraorbital and endonasal endoscopic approaches have gained favor; however there are limited published series comparing the use of these two techniques for these meningiomas. Using our patent database, we identified patients who underwent these two approaches, and conducted a retrospective chart review to compare outcomes between these two techniques.
Methods A total of 32 patients who underwent minimally invasive approaches were identified: 20 supraorbital and 11 endoscopic endonasal. Radiographic images, presenting complaints and outcomes, were analyzed retrospectively. The safety of each approach was evaluated.
Results The mean extent of resection through a supraorbital approach was significantly greater than that of the endoscopic endonasal approach, 88.1 vs. 57.9%, respectively ( p = 0.016). Overall, preoperative visual acuity and anopsia deficits were more frequent in the endonasal group that persisted postoperatively (visual acuity: p = 0.004; anopsia: p = 0.011). No major complications including cerebrospinal fluid (CSF) leaks or wound-related complications were identified in the supraorbital craniotomy group, while the endonasal group had two CSF leaks requiring lumbar drain placement. Length of stay was shorter in the supraorbital group (3.4 vs. 6.1 days, p < 0.001).
Conclusion Anterior skull base meningiomas can be successfully managed by both supraorbital and endoscopic endonasal approaches. Both approaches provide excellent direct access to tumor in carefully selected patients and are safe and efficient, but patient factors and symptoms should dictate the approach selected.
Keywords: supraorbital craniotomy, keyhole approaches, endonasal endoscopic, meningioma, anterior skull base, case series
Introduction
Meningioma is the most common primary brain tumor and arises from the arachnoid cap cells. 1 Meningiomas are very diverse in their location along the craniospinal axis and skull base meningiomas can be found anywhere from the crista galli to the foramen magnum. 1 Anterior skull base meningiomas most commonly involve the olfactory groove (OG), planum sphenoidale (PS), and/or the tuberculum sella (TS) and can extend further to invade the anterior clinoid process and parasellar regions. Meningiomas in these locations make up approximately 10% of intracranial meningiomas. 1 2 Upon discovery, these lesions can be large as they grow near “noneloquent” regions which often only cause subtle symptoms, such as frontal lobe compression, edema, and anosmia, as is seen in OG meningiomas. Alternatively, small lesions, such as tuberculum and planum meningiomas, can cause significant visual symptoms from compression of the optic apparatus.
The primary treatment modality of meningioma is surgery. The size, location, and presenting symptoms of the lesion all play an important role in selecting the approach taken. Since most meningiomas are benign, the goal of surgery is to achieve gross total resection without damaging the critical neurovascular structures to minimize tumor recurrence, which is inversely correlated with the extent of resection (EOR). 3
The traditional transcranial methods used to access these lesions include the pterional, subfrontal, and interhemispheric approaches with or without orbital osteotomies. 1 4 These approaches result in higher rates of gross total resection but can be associated with significant complications. 4 5 More recently, keyhole supraorbital 6 and expanded endoscopic approaches have been increasingly utilized to remove meningiomas in the anterior skull base. 7 These two approaches provide attractive, minimally invasive alternatives to traditional transcranial approaches in carefully selected patients where the primary goal of surgery, maximal safe resection, is not compromised.
Here, we present the results of our series of anterior skull base meningiomas where keyhole supraorbital and endoscopic approaches were used. We specifically compared outcomes in vision and the EOR, as well as other perioperative factors between each approach to further improve the decision-making process involved in choosing between these two approaches.
Methods
Patient Selection
A retrospective chart review from January 2005 to January 2020 was performed after receiving appropriate IRB approval. All patients included had procedures performed by the senior author (J.D.W.G.) and selection criteria were, a diagnosis of meningioma and procedural codes consistent with a supraorbital craniotomy with or without endoscopic assistance or an endonasal endoscopic approach. Basic demographic factors collected included age, gender, body mass index (BMI), and symptomology including visual acuity/anopsia deficits, and anosmia. Radiographic factors included tumor volume assessed by (A × B × C)/2 measurements and tumor location. To examine potential factors of ease of tumor resection (e.g., preoperative edema, brain–tumor interface) and risk of postoperative seizures, the presence or absence of abnormal adjacent fluid-attenuated inversion recovery (FLAIR) signal and diffusion-weighted imaging (DWI) were also collected. Operative features (use of lumbar drain, anticonvulsants, and skull base reconstruction method if an endonasal endoscopic approach was utilized) and postoperative outcomes (EOR, cerebrospinal fluid [CSF] leak, recurrence requiring subsequent surgery, postoperative radiation, length of stay, deep venous thrombosis [DVT], pulmonary embolism [PE], stroke, seizure, and diabetes insipidus [DI]) were recorded. Also, subsequent need for surgery or adjuvant radiation was recorded. In cases where postoperative radiation was administered, it was dictated by tumor grade and/or evidence of recurrence where surgery was not thought to provide meaningful cytoreductive potential.
Statistical Analysis
Statistical analysis was conducted with IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, New York, United States). Continuous variables were compared using a Student's t -test for normal variance and Welch's t -test for unequal variances and categorical variables were compared using a Chi-square test. A one-way analysis of variance (ANOVA) with post hoc Bonferroni's correction was used to compare the EOR by skull base location (OG, PS, and TS). Levene's test was used to assess for homogeneity of variances, and when homogeneity was violated, in place of the F-test, the Welch test was used. To assess neurologic deficits and postoperative features by tumor location effects, a 2 × 3 Chi-square test was performed and when appropriate, post hoc between group analyses with the Bonferroni's correction was conducted. To evaluate the effect of tumor volume on approach-related EOR, a linear regression analysis was performed adjusting for tumor volume and tumor location.
Supraorbital Surgical Technique
Video 1 Transciliary supraorbital craniotomy step-by-step technique.
The senior author (J.D.W.G.) performs the supraorbital craniotomy similar to previously published methodologies. 8 The head is elevated approximately 15 degrees and retroflexed 25 to 30 degrees. The head rotation is variable depending on the location, with 30 to 45 degrees contralateral rotation for lesions closer to the crista galli and more minimal rotation for lesions closer to the tuberculum. Navigation is utilized in all cases to confirm a satisfactory trajectory to the contralateral anterior and posterior pole of the tumor and minimize frontal sinus violation if possible. Microsurgery is utilized with 0- and 30-degree endoscopes (Olympus, Tokyo, Japan) also available; in our current series, they were used in 5/20 cases (25%). The abdomen is also prepped in the event where a fat graft is necessary. Incision is made in the upper-third of the eyebrow. Fine dissecting scissors are used to sharply dissect the orbicularis oculi and frontalis muscles to the subgaleal plane, and dissection above the pericranium is carried as far frontally as possible, so that a large pericranial graft may be harvested if necessary for dural reconstruction or repair of a frontal sinus defect. After the pericranium is incised frontally and dissected toward the orbital bar and flapped orbitally, single burr hole trephination at the keyhole directed toward the anterior fossa is performed. The craniotome is then used for first cutting as close as possible to the anterior fossa floor across the orbital bar stopping just lateral to the supraorbital foramen and lateral edge of the frontal sinus. The craniotome is then turned in a C -shaped fashion from the burr hole with as far frontal extension as possible and curved around to meet the medial edge of the basal cut, affording a bone flap approximately 4-cm wide by 2-cm tall. The inner edge of the orbital bar is then drilled flat extradurally to enhance visualization and minimize brain manipulation upon dural opening.
The dura is then opened in a C -shaped fashion based on the orbital bar. Protective telfa strips are then laid on the basal forebrain as dissection is taken to the optic nerve, optic cistern, and opticocarotid cistern for wide arachnoid dissection and CSF aspiration. Once sufficient brain relaxation is achieved and optic nerves and carotids identified, telfa sponges are placed over the optic nerves and carotids to identify and protect them and capsular dissection and internal debulking of the tumor proceeds. Once the tumor is resected, we frequently attempt to resect the dura and cauterize the underlying hyperostic bone either with a diamond burr and/or monopolar cautery; we do not typically resect the involved bone elements. Dura is closed primarily and bone flap replaced using two low-profile plates (Craniofix, Aesculap, Tuttlingen, Germany). The wound is closed in a multilayer fashion with a subcutaneous running nylon for skin closure that is left untied on either end with a compressive Kerlix head wrap for 48 hours to minimize soft tissue swelling. Suture removal is performed on postoperative day 5 (see Video 1 for step by step approach; available in the online version only).
Transnasal Surgical Technique
We routinely have the patient head 180 degrees from anesthesia with the head on a gel doughnut with the head turned 5 to 10 degrees to the right toward the operating surgeon. The otolaryngology team then prepares the nasal mucosa with afrin-soaked pledglets and lidocaine injection of the greater palatine foramen and posterior nasal septum. Turbinates are lateralized, sphenoidotomies are fashioned in the usual fashion, and the extent of sellar opening and posterior ethmoidectomy is dictated by the anterior extent of the tumor. Tumor location also dictates the extent of sellar, tuberculum, and planum drilling. For tuberculum meningiomas, bone decompression first begins in the superior sellar region, tuberculum, bilateral optic nerves and distal planum using the ultrasonic bone tips (Sonopet, Stryker, Portage, Michigan, United States). The dura and intercavernous sinus are then devascularized with bipolar cautery. After dural opening, resection begins with capsular dissection at the anterior margin of the tumor until the cortical interface is identified with internal debulking with the ultrasonic aspirator and taken posteriorly until inspection can reveal the optic nerves, chiasm, and anterior cerebral arteries. Thereafter, the sellar component of the tumor is internally debulked. At this point, careful arachnoidal dissection of the tumor from the optic nerves is performed until maximal safe resection is achieved.
After satisfactory tumor resection, the skull base is reconstructed. We utilize multilayer techniques. Most common is inlay/onlay duragen layers (Duragen Suturable, Integra, Plainsboro, New Jersey, United States) followed by epidurally positioned Sella con Resorb-x plate (Resorb-x plate, KLS Martin, Jacksonville, Florida, United States), gelfoam and merocel packing to be removed 5 to 7 days postoperatively. The resorb-x plate is used to provide a semirigid buttress, and when spanning the tuberculum, the plate is fashioned with an hourglass midsection shape to accommodate the carotid arteries and optic nerves in the hypotenuse angle between the planum/tuberculum and sella. Alternatively we use hydrated acellular dermal graft (Alloderm, LifeCell Corporation, Branchburg, New Jersey, United States) and either a resorb-x plate or surgicel-wrapped gelfoam (Surgicel, Johnson & Johnson, Somerville, New Jersey, United States; Gelfoam, Pfizer, New York, New York, United States) for gasket seal closure at the border of the defect (described previously as a single-layer “inlay/onlay” closure 9 ). We reserve a standard nasoseptal flap pedicled on the sphenopalatine artery for refractory leaks to avoid nasal morbidity.
Results
Thirty-one patients were identified that met inclusion criteria, 20 of which were treated via a supraorbital craniotomy and 11 via an endonasal endoscopic approach. Basic demographics are listed in Table 1 . In all patients, 59% had a preoperative visual acuity deficit, 62.5% had a field cut, and 15.6% had anosmia. Location distribution for OG, PS, and TS meningiomas was 8, 5, and 19 patients, respectively. There were two delayed postoperative CSF leaks 5 to 7 days after surgery when nasal packing was removed. Details of the transnasal approaches and reconstruction techniques are detailed in Table 2 .
Table 1. Overall patient demographics.
| Factor | Value |
|---|---|
| n | 31 |
| Mean age (SD) (years) | 59.1 (14.4) |
| Mean BMI (SD) (kg/m 2 ) | 31.8 (9.2) |
| Gender (M/F) | 9/22 |
| Supraorbital approach, n | 20 |
| Transnasal approach, n | 11 |
| Mean follow-up time in months (range) | 101.4 (1–380) |
Abbreviations: BMI, body mass index; F, female; M, male; SD, standard deviation.
Table 2. Transnasal approaches and reconstruction techniques.
| Location | Approach | Reconstruction technique |
|---|---|---|
| TS | Transsellar, transtubercular | Duragen, resporb-x plate |
| TS | Transsellar, transtubercular | Duragen, resorb-x, nasoseptal flap |
| TS | Transsellar, transtubercular | Alloderm, surgicel wrapped gelfoam |
| TS | Transsellar, transtubercular | Duragen, resorb-x plate |
| TS | Transsellar, transtubercular, transplanum | Nasoseptal flap |
| TS | Transsellar, transtubercular | Fat + nasoseptal flap |
| TS | Transsellar, transtubercular | Duragen, fat, duragen |
| TS | Transsellar, transtubercular | Alloderm, surgicel wrapped gelfoam, resorb-x |
| TS | Transsellar, transtubercular | Alloderm surgicel wrapped gelfoam, resorb-x plate |
| OG | Transethmoidal, transcribriform | External oblique fascia, fat, nasoseptal flap |
| TS | Transsellar, transtubercular, transplanum | Alloderm, resorb-x plate |
Abbreviations: OG, olfactory groove; TS, tuberculum sella.
A comparison of the findings between approaches is summarized in Table 3 . There was no difference in basic demographic features, tumor volume, or presenting deficits. Lumbar drains were only placed in the endonasal group. EOR was superior in the supraorbital group compared with the endonasal group. Postoperatively, visual acuity deficits and anopsia more frequently persisted in the endonasal group; this was likely driven by the larger proportion of preoperative vision deficits in the transnasal group (7/11 had both acuity and anopsia deficits compared with 12/20 acuity and 13/20 anopsia deficits in the supraorbital group) that were refractory to visual improvement (supraorbital treated 10/12 acuity and 9/13 anopsia deficits, while transnasal only had 1 patient's vision improve postoperatively). There were two CSF leaks, both of which occurred in the transnasal group. There were three patients with postoperative seizures, and all occurred in the supraorbital group. These patients presented after initial discharge for partial seizures but did not require long-term anticonvulsant medication. There were three clinically silent small peritumoral strokes in the supraorbital group. There was no association of preoperative FLAIR or postoperative FLAIR/DWI signal with stroke or seizures. Length of stay was statistically shorter in the supraorbital group.
Table 3. Differences between approaches.
| Factors | Supraorbital | Transnasal | p -Value |
|---|---|---|---|
| n | 20 | 11 | – |
| Mean age (y) | 60.7 | 56.0 | 0.382 |
| BMI (kg/m 2 ) | 30.5 | 34.2 | 0.299 |
| Female gender (%) | 15 (75%) | 7 (64%) | 0.505 |
| Pre-op tumor volume (cm 3 ) | 8.0 | 7.4 | 0.871 |
| Pre-op visual acuity deficit | 12 (63%) | 7 (64%) | 0.713 |
| Pre-op anopsia | 13 (68%) | 7 (64%) | 0.930 |
| Pre-op anosmia | 4 /16(25%) | 1/3 (33%) | N/A |
| Pre-op FLAIR change | 7 (35%) | 2 (18%) | 0.429 |
| No lateral or superior extension from CN II | 9 | 6 | – |
| Lateral extension from CN II | 2 | 2 | – |
| Superior extension from CN II | 5 | 3 | – |
| Lateral and superior extension from CN II | 4 | 0 | – |
| Anticonvulsant use | 12 (60%) | 2 (18%) | 0.057 |
| Lumbar drain use | 0 (0%) | 8 (73%) | <0.0001 |
| Post-op tumor volume (cm 3 ) | 0.2017 | 3.2 | 0.0439 |
| Extent of resection (%) | 88.1% | 57.9% | 0.016 |
| Post-op visual acuity deficit | 2 (10%) | 6 (54%) | 0.004 |
| Post-op anopsia | 4 (20%) | 6 (54%) | 0.011 |
| Post-op anosmia | 4/14 (29%) | 1/3 (33%) | N/A |
| Post-op seizure | 3 (15%) | 0 (0%) | 0.177 |
| Post-op radiographic stroke | 3 (15%) | 0 (0%) | 0.535 |
| CSF leak | 0 (0%) | 2 (18%) | 0.118 |
| Post-op FLAIR change | 7 (35%) | 4 (36%) | 1.000 |
| Post-op DWI signal | 7 (35%) | 0 (0%) | 0.033 |
| Length of stay (d) | 3.4 | 6.1 | <0.001 |
| Subsequent surgery for recurrence | 4 (20%) | 2 (18%) | 1.000 |
| Post-op radiation | 6 (30%) | 2 (18%) | 0.676 |
Abbreviations: BMI, body mass index; CN, cranial nerve; CSF, cerebrospinal fluid; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; N/A, not available; Post-op, postoperative; Pre-op, preoperative.
Further analysis was performed by stratifying patients by tumor location and is summarized in Table 4 . There were no differences in demographic features. One-way ANOVA showed a difference in preoperative tumor volume, and post hoc pairwise comparison revealed that OG were larger than both PS and TS meningiomas. Additionally, tumor location was associated with preoperative visual acuity deficits and anopsia, all occurring in TS lesions. Anticonvulsant prophylaxis was more frequently used in OG than tuberculum lesions proportionally. Tumor location had a strong trend with EOR with tuberculum lesions having the lowest. Persistent postoperative visual deficits were associated with a TS location, with more frequent visual acuity deficits and anopsia.
Table 4. Differences between locations.
| Factors | OG | PS | TS | p -Value (post hoc comparison p -value) |
|---|---|---|---|---|
| n | 8 | 4 | 19 | – |
| Mean age (y) | 56.8 | 57.5 | 60.3 | 0.838 |
| BMI (kg/m 2 ) | 26.8 | 32.4 | 33.8 | 0.201 |
| Female gender | 5 (62.5%) | 2 (50%) | 15(79%) | 0.423 |
| Pre-op tumor volume (cm 3 ) | 16.2 | 4.7 | 4.8 | 0.045 (OG vs TS p = 0.010, OG and PS p = 0.099) |
| Pre-op visual acuity deficit | 1 (12%) | 4 (100%) | 14 (74%) | 0.011 (OG vs TS p = 0.013) |
| Pre-op anopsia | 1 (12%) | 4 (100%) | 15 (79%) | 0.006 (OG vs TS p = 0.006) |
| Pre-op anosmia | 5 (62.5%) | 0/3 (0%) | 0/10 (0%) | N/A |
| Lumbar drain use | 1 (12%) | 0 (0%) | 7 (58%) | 0.188 |
| Anticonvulsant use | 7 (88%) | 1 (25%) | 6 (46%) | 0.020 (OG vs TS p = 0.024) |
| Extent of resection | 89.7% | 96.6.6% | 68.2% | 0.0976 |
| Post-op visual acuity deficit | 0 (0%) | 0 (0%) | 8 (62%) | 0.034 |
| Post-op anopsia | 0 (0%) | 0 (0%) | 10 (62%) | 0.003 |
| Post-op anosmia | 5 (62.5%) | 0 (0%) | 0 (0%) | <0.0001 |
| Post-op seizure | 1 (12%) | 0 (0%) | 2 (10%) | 0.772 |
| CSF leak | 1 (12%) | 0 (0%) | 1 (5%) | 0.669 |
| Subsequent surgery for recurrence | 2 (25%) | 1 (25%) | 3 (26%) | 0.819 |
| Post-op radiation | 1 (12%) | 2 (50%) | 5 (26%) | 0.374 |
Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; N/A, not available; OG, olfactory groove; Post-op, postoperative; Pre-op, preoperative; PS, planum sphenoidale; TS, tuberculum sellae.
To evaluate EOR by approach adjusted for preoperative tumor volume and location, a linear regression was performed with EOR as the dependent variable with approach, tumor volume, and location as independent variables. Approach alone was a significant predictor of EOR ( p = 0.026); location ( p = 0.575) and initial volume ( p = 0.613) were not significant. Mean EOR depicted by approach and location is shown in Table 5 . Representative cases are demonstrated in Figs. 1 , 2 , and 3 .
Table 5. Extent of resection by approach and location.
| Approach | Location | n (%) | Mean EOR (%) |
|---|---|---|---|
| Supraorbital | Olfactory groove | 7 | 89.6 |
| Planum sphenoidale | 4 | 96.6 | |
| Tuberculum sphenoidale | 9 | 83.3 | |
| Total | 20 | 88.1 | |
| Transnasal | Olfactory groove | 1 | 90.5 |
| Planum sphenoidale | 0 | N/A | |
| Tuberculum sphenoidale | 10 | 54.6 | |
| Total | 11 | 57.9 | |
| Totals | Olfactory groove | 8 | 90.0 |
| Planum sphenoidale | 4 | 96.6 | |
| Tuberculum sphenoidale | 19 | 68.9 | |
| Total | 31 | 77.4 |
Abbreviations: EOR, extent of resection; N/A, not available.
Fig. 1.
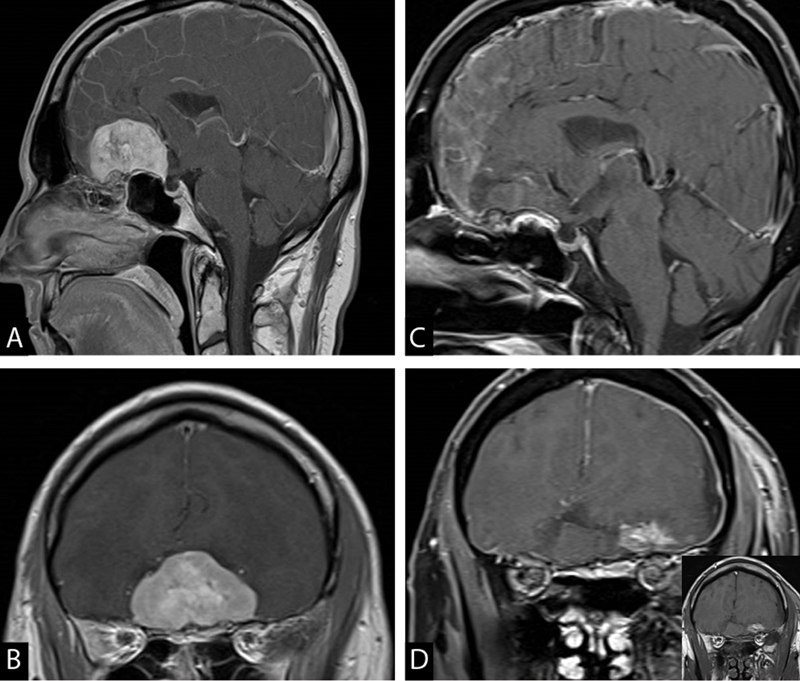
52-year-old female with past medical history of seizures and depression presented with confusion and was found to have a large olfactory groove meningioma in sagittal ( A ) and coronal ( B ) views. She was oriented to self and had anosmia on clinical exam. She underwent an uneventful keyhole supraorbital craniotomy for gross total resection of the tumor ( C and D ); the inset in ( D ) shows the noncontrasted T1 sequence indicating the enhancement is postoperative blood. Final pathology showed WHO grade II meningioma. She was discharged on postoperative day 3. She received 52.7 Gy radiation to tumor bed in 31 fractions. WHO, World Health Organization.
Fig. 2.
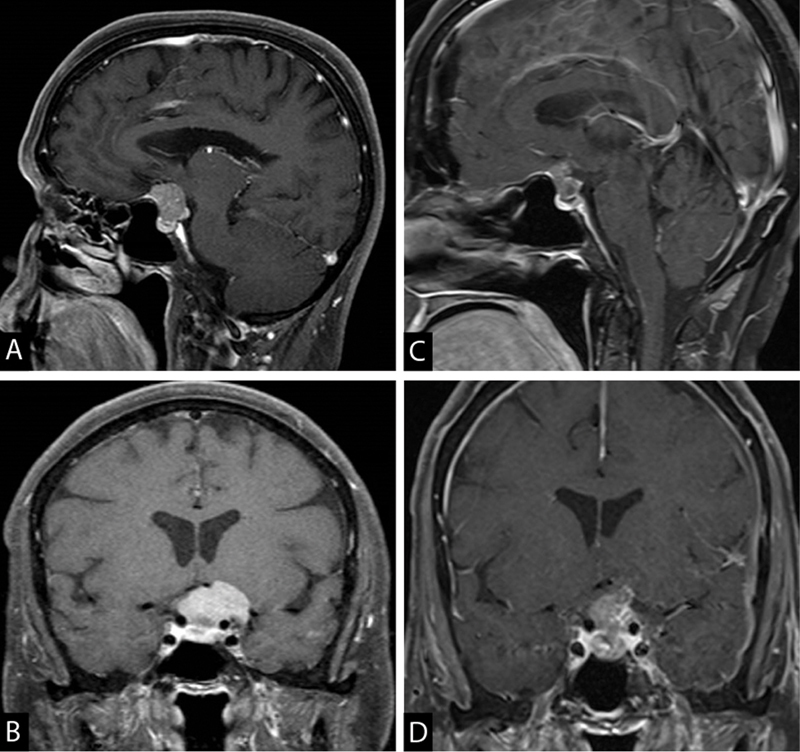
A 67-year-old female presented with visual field deficits and was found to have a TS meningioma with extension inferior to chiasmatic sulcus ( A ) and superolateral to the clinoidal carotid segment ( B ). She underwent planned subtotal resection via a supraorbital approach with a significant improvement in vision ( C, D ). Postoperatively, the patient received 52.7 Gy of radiation for the residual tumor. This case demonstrates the inferior limitation of the supraorbital approach for lesions extending below the chiasmatic sulcus, although it does provide better access to the tumor superior and lateral to the carotid. TS, tuberculum sella.
Fig. 3.
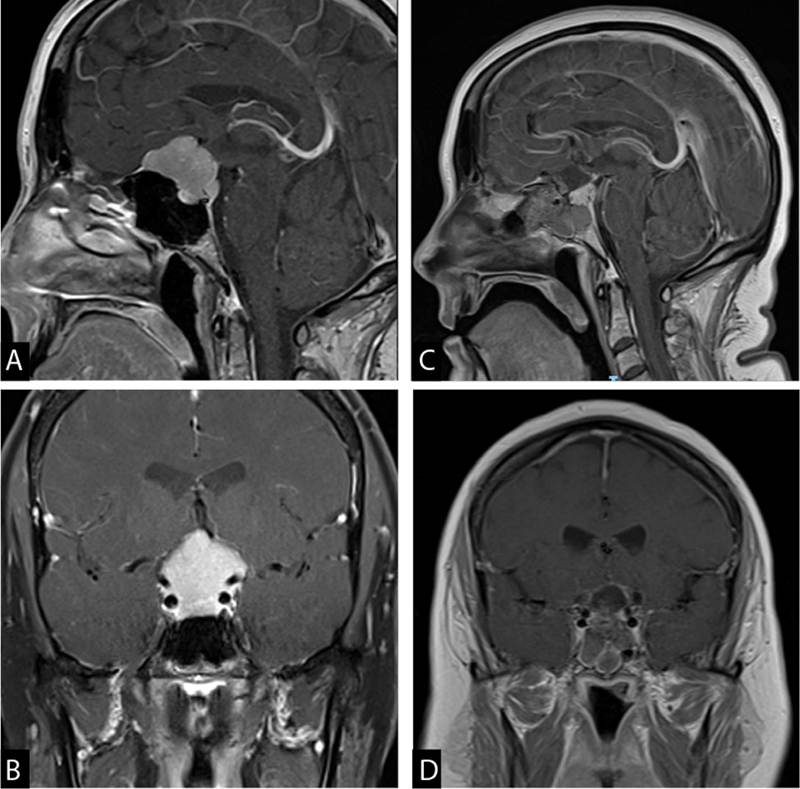
A 49-year-old female presented with progressively worsening visual field/acuity deficits for the past 2 months with mild headaches. She had total inferior temporal and nasal, and partial superior temporal and nasal visual field deficits in the left eye. In the right eye, she has partial inferior temporal and nasal and partial superior temporal field defects. MRI demonstrated a predominant tuberculum sella meningioma with extension to the planum ( A ) and suprasellar region ( B ). She underwent endoscopic, endonasal, transplanum, and transtuberculum near total resection of the mass ( C and D ). Final pathology was WHO grade-I meningioma ( Fig. 4B ). Patient had a postoperative CSF leak managed with a lumbar drain. Her visual field defects resolved postoperatively. CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; WHO, World Health Organization.
Given the relatively equal distribution of approaches for TS meningiomas (9 supraorbital vs. 10 transnasal), we did a follow-up subgroup analysis. Preoperative tumor volume in the supraorbital group was significantly smaller than the transnasal group (1.95 mm 3 ± 2.3 vs. 7.45 mm 3 ± 5.6, Welch's t -test p = 0.020). However, by using Fisher's exact test, there was no difference in preoperative visual acuity ( p = 1.000), preoperative anopsia ( p = 0.582), postoperative visual acuity ( p = 0.153), or postoperative anopsia ( p = 0.145). Adjusting for tumor volume, there was no difference in EOR by approach ( p = 0.106). Upon retrospective review, among the 10 patients in the transnasal group, 9 had lesions extending below the level of the chiasmatic sulcus and 1 remained above the chiasmatic sulcus. Conversely, in the supraorbital group, four extended below the chiasmatic sulcus and five remained above the chiasmatic sulcus. This distribution was nearly significant on Fisher's exact test ( p = 0.057). In the supraorbital group, there was no statistical difference in EOR ( p = 0.242), though those above the chiasmatic sulcus (92.9%) was greater than those below (71.2%).
Discussion
Along with the advancement of technology, cranial approaches are evolving. Better illumination, advances in surgical microscopes and navigation systems, and the continued development of microsurgical instruments have equipped neurosurgeons with tools that allow them to perform surgeries that are “more accurate, gentle, and safe.” 10 This has allowed skull base surgeons to perform patient-tailored approaches to prevent unnecessary brain exposure and retraction. The complications and effects of brain retraction are well established and multiple techniques have been developed to counter these effects including refined anesthetic techniques, patient positioning, and the use of lumbar drains and specialized brain retraction systems. 9 Furthermore, aggressive approaches, such as orbitozygomatic and bifrontal osteotomies, have been developed to minimize brain retraction during lengthy procedures. These techniques provide excellent exposure but can carry significant complications. 5 10 Retractorless dynamic surgery, seen with the minimally invasive supraorbital approach and transnasal endoscopic techniques, 8 11 12 has been an important advancement in protecting normal brain while accessing traditionally more difficult lesions. Ultimately, the outcomes of a surgical approach should not only be acceptable to the surgeon but also to the patient. As neurosurgical techniques continue to evolve, secondary but important cosmetic outcomes have acquired an increasing focus in addition to surgical outcomes. 13 14 Therefore, an informed discussion with the patient is warranted, comparing the risks and benefits of each approach.
In our series, we used the supraorbital approach in 20 patients with anterior skull base meningiomas. These included seven, four, and nine cases of OG), PS, and TS meningiomas, respectively. Mean EOR was 89.6% for OG, 96.6% for PS, and 83.3% for TS. Our complications from this approach are detailed in Table 3 and were similar to previously published series. 15 16 17 18 19 20 There were three postoperative seizures in the supraorbital group and none in the transnasal group despite all receiving antiepileptic drugs intraoperatively and postoperatively without correlation with preoperative FLAIR signal, postoperative FLAIR signal, or DWI signal.
Iacoangeli et al compared traditional craniotomies to the supraorbital approach in their series and did not find any significant difference in EOR or complications while showing improved cosmetic results. 19 In our series, excellent cosmetic results were achieved without any complications related to wound breakdown or significant muscle wasting on follow-up clinical evaluation, though this was assessed through clinic evaluation and not in a formal standardized fashion. Reisch et al in their series of 95 meningiomas resected using the supraorbital approach demonstrated that good cosmetic results contributed to increased patient satisfaction. 14 The supraorbital approach provides direct access to the lesion without significant muscle dissection and brain retraction but it does have limitations. 15 A cadaveric study by Borghei-Razavi et al showed that the sphenoethdmoidal suture is the limit of microsurgical drilling via supraorbital approach but further drilling of the superior two-thirds of the crista galli is possible with the use of an endoscope. 20 The endoscope is an excellent visualization tool that can complement the supraorbital approach and help overcome some of its limitations. At our institution, we always have an endoscope available during these cases to use as needed and utilized this adjunct in 25% of cases. It is important to review the patient-specific preoperative images in detail and understand the limitations of this approach as tailored to individual tumor or anatomical features. For instance, lesions involving the cribriform plate may be better suited for either a traditional craniotomy, expanded endonasal, or combined supraorbital and endonasal approaches. 15 20
The endoscopic endonasal approach provides another attractive minimally invasive approach to treat meningiomas in the anterior skull base. This approach offers the removal of bone involved with tumor, direct access to any extracranial portion of the tumor, no external scar, and early devascularization of the tumor. 15 21 In our current series of 11 patients, we treated 1 OG, 0 PS, and 10 TS meningiomas using an endoscopic approach. Mean EOR was 57.9% ranging from 90.0% for OG and 54.6% for TS as demonstrated in Table 5 . The primary goal of this technique in our series was tumor debulking and decompression of the optic apparatus to preserve vision. Complete macroscopic resection TS tumors is challenging, particularly when tumors extend lateral to the optic nerves and carotid arteries 22 ; as such subtotal resection is often the preoperative oncologic goal but still achieving goals of optic apparatus decompression. In our series, these lateral limits set by the orbit and important neurovascular structures including the optic nerves 7 21 dictated choice of approach. In some cases of TS tumors, we found the interface of the tumor and optic apparatus can be densely adherent precluding safe resection, whereas others have an identifiable and dissectible arachnoid plane ( Fig. 4 ).
Fig. 4.
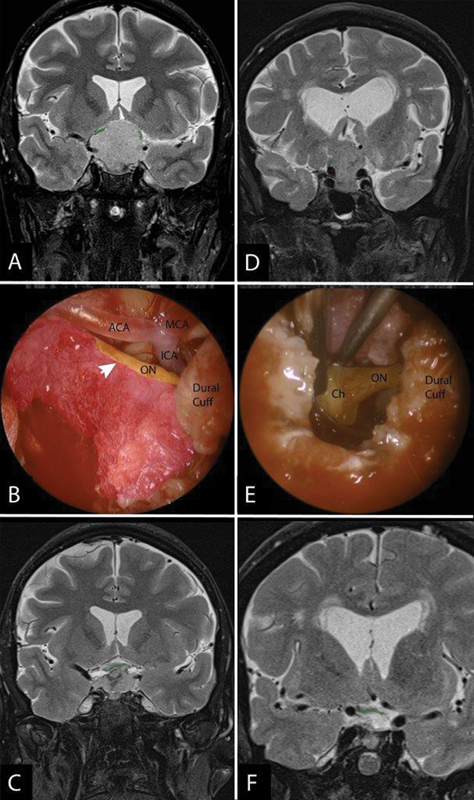
Variation of tumor-optic apparatus interface with illustrative cases. ( A-C ) A case of a tuberculum meningioma with displacement of the optic nerves superolaterally ( A , green tint) with intraoperative findings ( B ) showing tumor (pink tint) densely adherent to the optic nerve (arrowhead). Given this adherence to the optic apparatus and hypophyseal branches, residual was left behind so as to not exacerbate the patient's visual deficit by being overly aggressive and avulsing the nerve but still achieving optic apparatus decompression ( C ). Conversely, ( D-F ) shows a different tuberculum meningioma with superolateral displacement of the optic nerves ( D ) that intraoperatively had a far more dissectible tumor from the optic nerve (ON) and Chiasm (Ch) ( E ). As a result, a GTR was achieved ( F ). GTR, gross total resection.
For our patients treated with transnasal surgery, CSF leak was noted in only two (6.3%) cases but preoperative lumbar drain was placed in eight (25%) patients. In a series of 35 patients with anterior skull base meningiomas, Gardner et al had a CSF leak rate of 40% using an endoscopic endonasal approach. 7 With the use of nasoseptal vascularized flaps, this rate has been reduced significantly and a recently published case series reported a rate of 17.6%. 23 It is likely that higher CSF leak rates reflect larger skull base defects incurred from expanded endonasal approaches (EEA) required to address larger tumors. The benefit of an EEA may be a higher EOR rate as demonstrated by Gardner et al. 7 Even though CSF leak rates have declined over the years with the endoscopic endonasal approach, they remain significantly higher than traditional transcranial and keyhole supraorbital approaches, 4 which our data supports as well. A meta-analysis by Musken et al. supports that transcranial approaches have higher EOR but interestingly, the endoscopic approaches carry a higher rate of visual improvement. 4
Supraorbital and endonasal endoscopic approaches usually provide adequate corridors for access to tumors in the anterior skull base. In carefully selected patients, these approaches can provide direct access to the lesion, and minimize brain retraction. There are published algorithms proposed for patient selection for either approach. 24 25 These algorithms include lateral extension beyond the internal carotid artery, optic nerve and lamina papyracea, olfactory status, and involvement of cribriform plate. 22 When deciding on an approach, we largely follow a similar algorithm. A supraorbital approach was more frequently selected if the tumor extended lateral to and/or capped the optic nerve, as satisfactory decompression via a transnasal approach in such instances would be unlikely. However, in some cases the transnasal approach was chosen despite tumor extent superior or lateral to the optic nerves, as we thought, it would best accomplish the goals of surgery. Though our postoperative visual outcomes favor the supraorbital approach, this analysis is limited as we approached fewer cases endonasally. Moreover, the transnasal approach tended to be selected in cases with larger TS lesions with more threatened vision where the main goal of surgery is to save or preserve vision; this may bias the between approach EOR analysis and postoperative vision outcomes. Detailed neuroophthalmological data including preoperative degree of optic atrophy and retinal nerve fiber layer thinning informs expectations for degree of potential visual improvement following surgical decompression. Unfortunately, this data was not available for all of our patients.
Our data also reflects a surgical bias toward transcranial routes to these meningiomas in our center. Some of this bias is a result of a relatively high mean BMI that is associated with a higher CSF leak rate from the transnasal corridor. 26 This patient body habitus factor contributes to use of an additional perioperative procedure (i.e., lumbar drain) and longer in-hospital stay compared with our supraorbital alternative. In addition, more extensive and more anterior lesions at the anterior skull base approached via transnasal approaches will likely result in higher rates of anosmia compared with supraorbital resections in those patients with preserved olfaction preoperatively. Our small number of patients does not allow direct comparisons of this feature here, and retrospective studies may not accurately assess olfactory function if documentation is insufficient.
EOR, vision outcomes, and CSF leak rates in these anterior skull base lesions will likely vary by center and surgeon based on training and experience. 27 While an algorithm helps quantify the advantages, risks, and benefits of these minimally invasive approaches, cases with clinical equipoise ought to be approached via the corridor that the surgeon thinks will achieve the best results informed by their own experience. In our admittedly limited analysis, it appears that TS lesions that largely remain above the chiasmatic sulcus had better EOR approached via a supraorbital route while lesions with subchiasmal extent tended to have residual, though this was not statistically significant. In this scenario, a combined or staged supraorbital and endoscopic approach may be helpful in achieving gross total resection. 15 This may spare the need to repair larger skull base defects if a purely transnasal endoscopic approach was utilized with the expanded transplanum and transtuberculum corridors that have associated CSF leak risks and nasal morbidity. 28 29 30 31
Some anterior skull base meningiomas will remain not suitable for either of these minimally invasive routes and still require traditional transcranial surgery. Liu et al noted in their review of OG meningiomas that those approached from a transbasal approach tended to have the greatest tumor volume, cerebral edema, and vascular involvement. 32 As a result, very large tumors, for example, are absent from our series here. Additional factors that must be considered in patient selection include, age, overall health status, prior radiation, and hairline. We conducted a detailed discussion with the patient, evaluating the risks and benefits of each approach, and strongly consider the patients' wishes in our decision making. Only after eliciting an accurate history, physical examination, radiographic review, and discussion with the patient will these goals be determined, whether it is gross total resection, maximal safe resection, or vision preservation. In most cases, the surgical corridor will be determined; at clinical equipoise, the experience and judgment of the surgeon should prevail.
Conclusion
Supraorbital and endonasal endoscopic approaches provide safe and cosmetically favorable approaches to meningioma resection in the anterior skull base. These approaches should not be considered as a replacement to the traditional open approaches but as additional options that a skull base surgeon can effectively utilize in carefully selected patients.
Funding Statement
Funding None.
Conflict of Interest None declared.
Note
This work has not been previously published or presented at the time of submission.
References
- 1.Rami Almefty G FH, Ossama Al-Mefty.Meningioma7th edPhiladelphia, PA: Elsevier; 20171107–1132. [Google Scholar]
- 2.Abbassy M, Woodard T D, Sindwani R, Recinos P F. An overview of anterior skull base meningiomas and the endoscopic endonasal approach. Otolaryngol Clin North Am. 2016;49(01):141–152. doi: 10.1016/j.otc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Nanda A, Bir S C, Maiti T K, Konar S K, Missios S, Guthikonda B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg. 2017;126(01):201–211. doi: 10.3171/2016.1.JNS151842. [DOI] [PubMed] [Google Scholar]
- 4.Muskens I S, Briceno V, Ouwehand T L. The endoscopic endonasal approach is not superior to the microscopic transcranial approach for anterior skull base meningiomas-a meta-analysis. Acta Neurochir (Wien) 2018;160(01):59–75. doi: 10.1007/s00701-017-3390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura M, Struck M, Roser F, Vorkapic P, Samii M.Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach Neurosurgery 20076005844–852., discussion 844–852 [DOI] [PubMed] [Google Scholar]
- 6.Ormond D R, Hadjipanayis C G. The supraorbital keyhole craniotomy through an eyebrow incision: its origins and evolution. Minim Invasive Surg. 2013;2013:296469. doi: 10.1155/2013/296469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner P A, Kassam A B, Thomas A.Endoscopic endonasal resection of anterior cranial base meningiomas Neurosurgery 2008630136–52., discussion 52–54 [DOI] [PubMed] [Google Scholar]
- 8.Gazzeri R, Nishiyama Y, Teo C. Endoscopic supraorbital eyebrow approach for the surgical treatment of extraaxial and intraaxial tumors. Neurosurg Focus. 2014;37(04):E20. doi: 10.3171/2014.7.FOCUS14203. [DOI] [PubMed] [Google Scholar]
- 9.Casiano R R. Philadelphia, PA: Wolters Kluwer; 2015. Endonasal resection of the anterior cranial base; pp. 173–183. [Google Scholar]
- 10.Matsushima T, Kobayashi S, Inoue T, Rhoton A S, Vlasak A L, Oliveira E. Albert L. Rhoton Jr., MD: his philosophy and education of neurosurgeons. Neurol Med Chir (Tokyo) 2018;58(07):279–289. doi: 10.2176/nmc.ra.2018-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews R J, Bringas J R.A review of brain retraction and recommendations for minimizing intraoperative brain injury Neurosurgery 199333061052–1063., discussion 1063–1064 [DOI] [PubMed] [Google Scholar]
- 12.Dehdashti A R, Ganna A, Witterick I, Gentili F.Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations Neurosurgery 20096404677–687., discussion 687–689 [DOI] [PubMed] [Google Scholar]
- 13.Boari N, Spina A, Giudice L, Gorgoni F, Bailo M, Mortini P. Fronto-orbitozygomatic approach: functional and cosmetic outcomes in a series of 169 patients. J Neurosurg. 2018;128(02):466–474. doi: 10.3171/2016.9.JNS16622. [DOI] [PubMed] [Google Scholar]
- 14.Reisch R, Marcus H J, Hugelshofer M, Koechlin N O, Stadie A, Kockro R A. Patients' cosmetic satisfaction, pain, and functional outcomes after supraorbital craniotomy through an eyebrow incision. J Neurosurg. 2014;121(03):730–734. doi: 10.3171/2014.4.JNS13787. [DOI] [PubMed] [Google Scholar]
- 15.Banu M A, Mehta A, Ottenhausen M. Endoscope-assisted endonasal versus supraorbital keyhole resection of olfactory groove meningiomas: comparison and combination of 2 minimally invasive approaches. J Neurosurg. 2016;124(03):605–620. doi: 10.3171/2015.1.JNS141884. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y J, Chen K T, Lee C C. Anterior skull base tumor resection by transciliary supraorbital keyhole craniotomy: a single institutional experience. World Neurosurg. 2018;111:e863–e870. doi: 10.1016/j.wneu.2017.12.177. [DOI] [PubMed] [Google Scholar]
- 17.Lucas J W, Zada G. Endoscopic endonasal and keyhole surgery for the management of skull base meningiomas. Neurosurg Clin N Am. 2016;27(02):207–214. doi: 10.1016/j.nec.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Cai M, Hou B, Luo L, Zhang B, Guo Y. Trans-eyebrow supraorbital keyhole approach to tuberculum sellae meningiomas: a series of 30 cases with long-term visual outcomes and recurrence rates. J Neurooncol. 2019;142(03):545–555. doi: 10.1007/s11060-019-03128-9. [DOI] [PubMed] [Google Scholar]
- 19.Iacoangeli M, Nocchi N, Nasi D. Minimally invasive supraorbital key-hole approach for the treatment of anterior cranial fossa meningiomas. Neurol Med Chir (Tokyo) 2016;56(04):180–185. doi: 10.2176/nmc.oa.2015-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borghei-Razavi H, Truong H Q, Fernandes-Cabral D T. Minimally invasive approaches for anterior skull base meningiomas: supraorbital eyebrow, endoscopic endonasal, or a combination of both? anatomic study, limitations, and surgical application. World Neurosurg. 2018;112:e666–e674. doi: 10.1016/j.wneu.2018.01.119. [DOI] [PubMed] [Google Scholar]
- 21.Hayhurst C, Teo C.Tuberculum sella meningioma Otolaryngol Clin North Am 20114404953–963., viii–ix [DOI] [PubMed] [Google Scholar]
- 22.Youngerman B E, Banu M A, Gerges M M. Endoscopic endonasal approach for suprasellar meningiomas: introduction of a new scoring system to predict extent of resection and assist in case selection with long-term outcome data. J Neurosurg. 2020;135(01):113–125. doi: 10.3171/2020.4.JNS20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoli M, Guaraldi F, Pasquini E, Frank G, Mazzatenta D. The Endoscopic endonasal management of anterior skull base meningiomas. J Neurol Surg B Skull Base. 2018;79 04:S300–S310. doi: 10.1055/s-0038-1669463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kshettry V R, Elshazly K, Evans J J. Endoscopic transnasal surgery for planum and tuberculum sella meningiomas: decision-making, technique and outcomes. CNS Oncol. 2016;5(04):211–222. doi: 10.2217/cns-2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ottenhausen M, Rumalla K, Alalade A F. Decision-making algorithm for minimally invasive approaches to anterior skull base meningiomas. Neurosurg Focus. 2018;44(04):E7. doi: 10.3171/2018.1.FOCUS17734. [DOI] [PubMed] [Google Scholar]
- 26.Dlouhy B J, Madhavan K, Clinger J D. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg. 2012;116(06):1311–1317. doi: 10.3171/2012.2.JNS111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Younus I, Gerges M M, Uribe-Cardenas R. How long is the tail end of the learning curve? Results from 1000 consecutive endoscopic endonasal skull base cases following the initial 200 cases. J Neurosurg. 2020;134(03):750–760. doi: 10.3171/2019.12.JNS192600. [DOI] [PubMed] [Google Scholar]
- 28.Soudry E, Psaltis A J, Lee K H, Vaezafshar R, Nayak J V, Hwang P H. Complications associated with the pedicled nasoseptal flap for skull base reconstruction. Laryngoscope. 2015;125(01):80–85. doi: 10.1002/lary.24863. [DOI] [PubMed] [Google Scholar]
- 29.Lavigne P, Faden D L, Wang E W, Snyderman C H. Complications of nasoseptal flap reconstruction: a systematic review. J Neurol Surg B Skull Base. 2018;79 04:S291–S299. doi: 10.1055/s-0038-1668158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wengier A, Ram Z, Warshavsky A, Margalit N, Fliss D M, Abergel A. Endoscopic skull base reconstruction with the nasoseptal flap: complications and risk factors. Eur Arch Otorhinolaryngol. 2019;276(09):2491–2498. doi: 10.1007/s00405-019-05531-4. [DOI] [PubMed] [Google Scholar]
- 31.Jalessi M, Jahanbakhshi A, Amini E, Kamrava S K, Farhadi M. Impact of nasoseptal flap elevation on sinonasal quality of life in endoscopic endonasal approach to pituitary adenomas. Eur Arch Otorhinolaryngol. 2016;273(05):1199–1205. doi: 10.1007/s00405-015-3729-z. [DOI] [PubMed] [Google Scholar]
- 32.Liu J K, Silva N A, Sevak I A, Eloy J A. Transbasal versus endoscopic endonasal versus combined approaches for olfactory groove meningiomas: importance of approach selection. Neurosurg Focus. 2018;44(04):E8. doi: 10.3171/2018.1.FOCUS17722. [DOI] [PubMed] [Google Scholar]


