FIGURE 3.
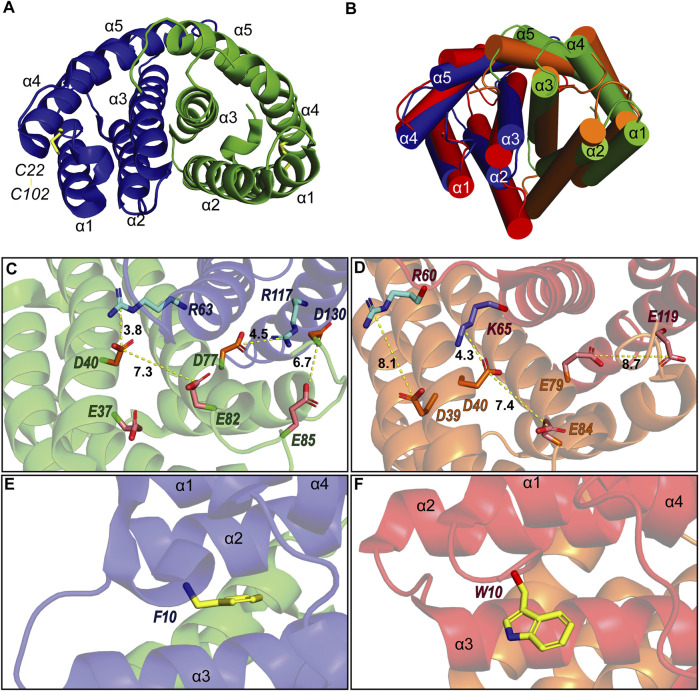
(A) Structure of A. argentata TuSp NT dimer (PDB ID 6TV5) with the two subunits shown in blue and green and their helices numbered. The intramolecular disulfide is highlighted with yellow stick representation. (B) Superposition of the structures of TuSp NT and E. australis MaSp NT (PDB ID 2LTH, red) dimers. α-helices are shown as cylinders. (C) and (D) shows charge interactions between residues on the subunit interface for A. argentata TuSp (C) and E. australis MaSp (D) NT, respectively. Aspartic acid side chains are colored orange, glutamic acids are pink, arginines are cyan and lysines are blue. (E) and (F) show the orientation of F10/W10 side chain within each structure (yellow).