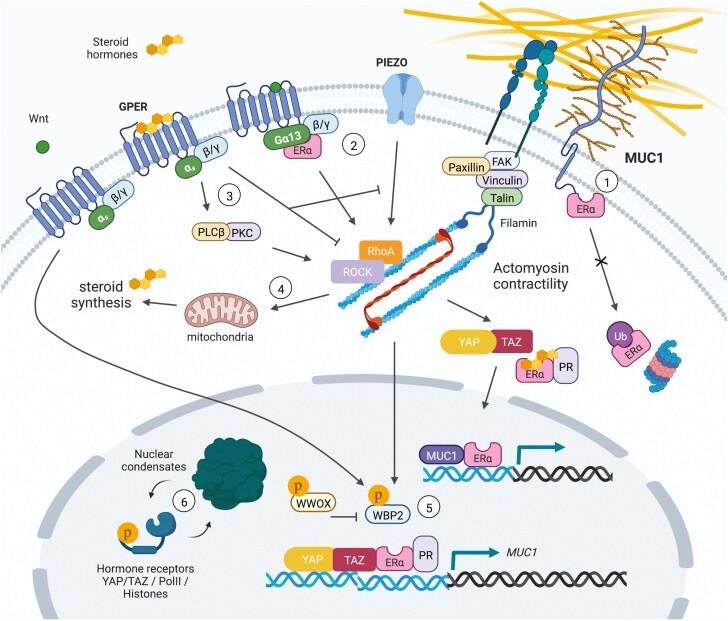
Figure 2.
Mechanosensitive hormone signaling associated with membrane/cytoskeletal dynamics and transcription factor activity. (1) The glycoprotein MUC1 directly interacts with ERα to inhibit its ubiquitination and degradation, and binds with ERα at estrogen-responsive gene promoters to stimulate ERα-mediated transcription. Steroid hormones can also stimulate MUC1 expression. (2) Estrogen induces rapid actin cytoskeleton and membrane remodeling through ERα-Gα13 interactions upstream of RhoA/ROCK/moesin signaling. (3) GPER links estrogen stimulation to PLCβ-PKC and RhoA/ROCK induced actin assembly to drive mechanosensitive TAZ activation and cell migration. GPER can alternatively dampen mechanical stress induced RhoA activity and actin polymerization, resulting in inhibition of apoptosis induced by the mechanosensitive ion channel, Piezo1, as a protective mechanism against cartilage degeneration. (4) RhoA activity is required in PR-expressing luteal cells of the corpus luteum to maintain mitochondrial density, efficient cholesterol transport, and progesterone synthesis. In skeletal muscle cells, mechanical stretch and testosterone stimulation results in increased expression of estrogen-related synthases and estrogen production. (5) WBP2 is a cofactor for ERα/PR and YAP/TAZ transcriptional activity. Wnt signaling suppresses ubiquitin mediated degradation of WBP2 to promote breast cancer progression, whereas phosphorylation of the tumor suppressor, WWOX, triggers its binding to WBP2 to inhibit ERα-mediated transcription. Alternatively, mechanosensitive tyrosine kinase signaling can phosphorylate WBP2 to enhance ERα-mediated transcription. (6) As observed for YAP/TAZ, the intrinsically disordered regions of hormone receptors (AR/PR) could allow for mechanosensitive phase separation and condensate formation to govern hormone-induced transcriptional complex activity.