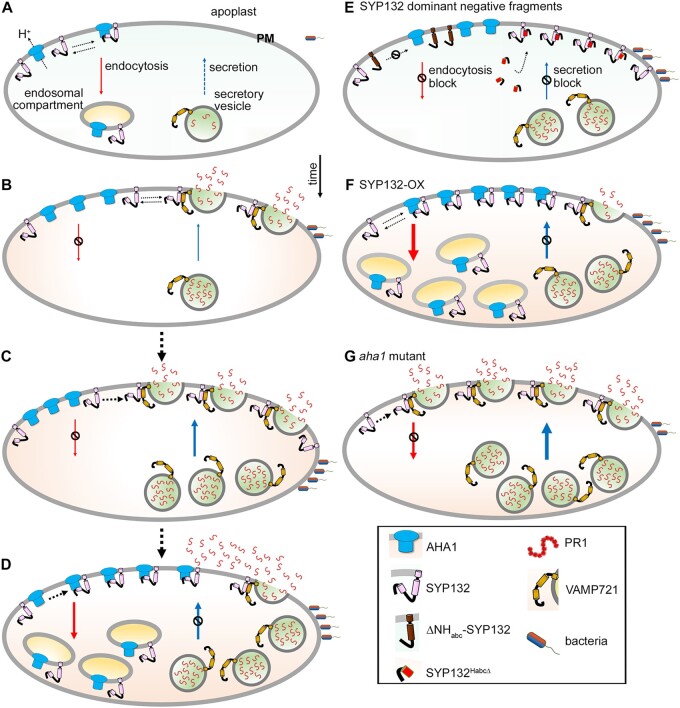
Figure 7.
SNARE SYP132 mediates divergent trafficking pathways of PM H+-ATPase AHA1 and PR1 during bacterial pathogenesis. Schematic model depicting a snapshot of the events for SYP132, AHA1, and PR1 that follow pathogen infection. A, Regulation of proton (H+) ATPase density and function at the plasma membrane is mediated by the trafficking SNARE SYP132 which binds AHA1 and promotes its endocytosis. B–D, During bacterial pathogenesis co-ordinated SYP132 and AHA1 traffic regulates SNARE density and functions. SYP132 is diverted for antimicrobial PR1 secretion essential for pathogen defenses, and consequently AHA1 endocytosis is suppressed (B). SYP132 expression increases and supports the increasing demand for PR1 secretion (C) AHA1 abundance increases and as interactions between AHA1 and SYP132 are enhanced co-ordinate endocytic trafficking of SYP132 with AHA1 moderates SYP132 density and PR1 secretion (D). E, Over-expression of dominant-negative ΔNHabc-SYP132 lacking the critical binding (Habc) domain titrates out the full-length SYP132 and prevents SYP132–AHA1 interactions to block AHA1 endocytosis. The dominant-negative SYP132 peptide (SYP132HabcΔ) lacks the Qa-SNARE motif but retains the Habc domain that actively binds full-length SNARE proteins preventing normal SYP132 functions. Over-expression of the SYP132HabcΔ therefore blocks AHA1 endocytosis and inhibits PR1 secretion. F, SYP132 overexpression (SYP132-OX) promotes both AHA1 and SYP132 internalization which blocks PR1 secretion for pathogen defense. G, In the Arabidopsis aha1-7 mutant, AHA1-dependent SYP132 endocytosis is blocked. Consequently, more SNARE is available for secretory traffic at the plasma membrane and PR1 accumulation in the apoplast is significantly enhanced. Box (bottom right) is the key to the schematic. Thicker arrows indicate increased trafficking. Bacterial infection and the secretory vesicle localized SYP132 SNARE-complex partner VAMP721 are shown. Other secretory SNARE-complex components are not shown for simplicity. Sizes are not to scale.