Abstract
Introduction:
Cure rates for pediatric and young adult patients with refractory or recurrently relapsed acute lymphoblastic leukemia (ALL) are dismal. Survival from time of relapse is typically measured in weeks to months, and standard chemotherapy and currently approved targeted therapy achieve remission in less than a third of affected patients. To date, the only definitive curative therapy has been allogeneic hematopoietic stem cell transplant (HSCT). Advances in immunotherapy, with the introduction of chimeric antigen receptor T-cell therapies and the development of tisagenlecleucel, have changed the landscape.
Areas covered:
This review will describe the pharmacology of tisagenlecleucel and summarize the clinical evidence for its use in the treatment of multiple-relapsed or refractory B-cell ALL (B-ALL). Also discussed are other immunotherapies for B-ALL as well as the most commonly-encountered toxicities and corresponding management strategies.
Expert commentary:
Early phase trials indicate that tisagenlecleucel significantly improves survival for patients with B-ALL that is refractory or in second or later relapse. In responding patients, remissions have been reported on the order of years, and thus, tisagenlecleucel may herald a dramatic shift in the treatment paradigm of this largely fatal disease.
Keywords: Tisagenlecleucel, cytokine release syndrome, CD19, Kymriah, lymphoblastic leukemia, chimeric antigen receptor T cell, CART, immunotherapy
1. Introduction
Approximately 13000 new cases of acute lymphoblastic leukemia (ALL) are diagnosed in the United States [1] and Europe [2] annually. The burden of ALL primarily lies with the young: approximately two thirds of cases are diagnosed in patients younger than 34 years old, and the vast majority of those (85%) are diagnosed before age 20 [1]. As such, it is the most common cause of cancer in children. For adults diagnosed with ALL, the 5-year survival rates are low, approximately 40%, and among those diagnosed over the age of 60, the five year survival rate is only 10% [3]. In contrast, overall survival for children diagnosed with ALL now approaches 90%. This success is driven primarily by advances in the treatment of de novo ALL. In contrast, only one third of children survive disease recurrence [4]. As a result, leukemia remains a leading cause of non-accidental death in childhood.
The diagnosis of ALL encompasses a variety of disease types. The B-precursor cell phenotype, characterized by the presence of B-cell-associated antigens such as CD9, CD19, CD22, and CD79a, accounts for 80–85% of pediatric ALL [5]. Other subtypes, beyond the scope of this article, include mature B-cell ALL and T-cell ALL [5].
The initial approach to treatment of ALL in children incorporates multiple chemotherapeutics applied in a risk-adapted manner. The intensity of frontline therapy is dictated by clinical features (age, white blood cell count at diagnosis, presence or absence of central nervous system disease), biological characteristics (immunophenotype, cytogenetics, sentinel genetic alterations), and response to therapy measured by morphology and minimal residual disease (MRD). Standard therapy is administered over 2.5–3.5 years, with 9 months of intensive therapy followed by 2–3 years of less intensive, ‘maintenance’ therapy.
In total, 10–20% of children relapse after or do not respond to frontline therapy [4]. Among those who relapse, overall survival rates depend on the length of the first complete remission (those relapsing within 36 months of initial diagnosis have a worse prognosis) and site of relapse (isolated extramedullary disease is associated with favorable outcomes) [6]. Those who experience late, isolated extramedullary relapses have the best prognosis, with event-free survival rates of approximately 80% when treated with intensive chemotherapy and radiation [5]. But despite the relative success with late extramedullary relapses, those who relapse early with medullary disease continue to have a dismal prognosis, with 5-year survival rates of 10–20% [6]. Refractory ALL portends an equally poor prognosis with a 10 year overall survival of approximately 30% [7]. The likelihood of achieving a clinical remission and long-term survival with subsequent relapses is poorer still [4]. As with initial therapy, a risk-adapted approach to treatment of relapsed and refractory ALL is utilized, though stratified on different variables––immunophenotype, site of relapse, and length of initial remission––and therapy may include intensive multidrug chemotherapy, radiation and/or HSCT [8].
Tisagenlecleucel (KYMRIAH®, Novartis Pharmaceuticals Corporation), an immunotherapy that was recently approved by the US Food and Drug Administration (FDA) for the treatment of patients younger than 25 years old with B-cell ALL that is refractory or in second or later relapse [9], has introduced a new treatment option for this population.
2. Overview of the market
Anti-leukemia treatment has evolved from initial monotherapy that induced the first disease responses in the late 1940s to short-course multi-agent therapy that conferred brief remissions, to multi-agent therapy in ‘blocks’ pioneered in the 1960s that continue to function as the backbone of anti-leukemia therapy today [10]. The therapy for relapse has similarly evolved. Initially, relapse reinduction was composed of re-exposure to agents used in the initial control of the disease, now it is typically comprised of multiple blocks of intensive chemotherapy that can include more than 10 individual cytotoxic drugs with or without cranial radiation [11-13]. Success seen in one such regimen, studied in the UK ALLR3 trial, with estimated 3-year progression-free survival of 64.6% (54.2–73.2) and 3-year overall survival of 69% (58.5–77.3) [11] has made UK R3 a preferred regimen for relapsed ALL in children and young adult. Acute toxicity of this regimen is high, with nearly all patients experiencing at least one Grade ≥ 3 non-hematologic adverse event during UK R3 reinduction [14]and with each relapse, patients are at increased risk of sequelae and toxicity associated with earlier intensive therapy [13]. Moreover, the toxicity of the UKR3 backbone when combined with novel agents is often too high [14]. Other similar but less intensive multi-agent backbones are also often used in the relapsed setting in trials integrating novel agents.
Two additional cytotoxic chemotherapeutic agents have been developed and are specifically approved for use in the relapsed and refractory setting: vincristine sulfate liposomal injection has been labeled by the FDA for the treatment of adult relapsed ALL [15,16], and clofarabine, which was approved by the FDA in 2004 for use in pediatric patients with relapsed or refractory ALL and two prior treatment regimen failures [17]. Unfortunately the addition of these agents only induces a complete remission in 20% of patients [18-20]. The only curative therapy for recurrent or high-risk relapse in pediatric ALL to date has been allogeneic HSCT. The success of this therapy depends on the patient achieving a complete clinical remission, with improved outcomes if the patient is MRD negative prior to HSCT [21].
Further intensification of cytotoxic chemotherapy is unlikely to improve survival in relapsed ALL [22]. Thus, the development of novel agents and targeted therapies is essential. Immunotherapy, consisting of monoclonal antibodies and chimeric antigen receptor T-cell therapy, has therefore stepped to the forefront. There are several types of antibody agents currently utilized in anti-leukemia therapy. Monoclonal anti-bodies are designed to bind to a target that is abundantly present on malignant cells, but less expressed on normal cells, thus limiting impact on healthy tissue. Antibodies can be naked or conjugated to a cytotoxic agent. After binding to the target antigen, it is internalized by the cell and the cytotoxic drug is released, providing an additional mechanism for leukemic-targeted killing. Finally, bispecific T-cell engager (BiTE) antibodies are antibodies that contain two binding sites to engage the patient’s own immune system to target malignant cells [23].
Two novel immunotherapies have recently demonstrated considerable efficacy for relapsed or refractory B-ALL. Blinatumomab (BLINCYTO®, Amgen Inc.) and inotuzumab ozogamicin (Besponsa®, Pfizer Inc.), have been approved by both the FDA [24,25] and the European Medicines Agency (EMA) [26,27] for the treatment of relapsed and refractory ALL in adults. In 2017, the FDA extended blinatumomab’s labeling indication to include pediatric use [25], and was extended again in 2018 to include treatment of B-cell precursor ALL in first or second complete remission with MRD greater than or equal to 0.1% [28]. Blinatumomab, a BiTE, functions by linking CD19+ lymphoblasts with the patient’s CD3+ T cells, thereby activating the T cells to kill the leukemia cells [23]. This therapy has an approximately 40% complete response (CR) rate in adult trials for patients who were primary refractory after induction, who had relapsed within a year of first remission or allogeneic HSCT, or were in second or later relapse [29,30]. In children, a 39–67% overall response rate (CR, or CR with incomplete hematologic recovery) has been seen in Phase I/II trials for patients with refractory disease or in second or later relapse or in evaluation of ‘compassionate use’ [8,31,32]. One quarter to one half of both pediatric and adult patients proceed to HSCT after achieving CR with blinatumomab [8,29-31]. The median overall survival after treatment with blinatumomab on these trials in is 6.1–7.3 months [29,30] and 7.5 months in adults and pediatrics, respectively [31]. The pivotal Phase-III trial, treating adult patients with relapsed or refractory CD22-positive ALL with inotuzumab ozogamicin, an anti-CD22 antibody conjugated to cytotoxic calicheamicin [23,33], demonstrated a high overall response rate of 80.7% (95% CI 72.1–87.7), but the median remission duration was only 4.6 months and the overall survival was 7.7 months [30]. Although there are no completed pediatric trials for inotuzumab, analysis of the compassionate use program in the US showed a 62% CR rate [8], and a current Children’s Oncology Group Phase-III trial is ongoing (ClinicalTrials.gov NCT02981628).
Thus, with no clear superior agent, the treatment of relapsed and refractory ALL in pediatric and young adult patients is based on local institutional guidelines and physician and family preference, using these agents and other standard chemotherapies, to achieve remission with intent to proceed to HSCT if an appropriate donor is available. Tisagenlecleucel, a chimeric antigen receptor T-cell therapy, described extensively below, offers an additional modality of treatment in this setting.
3. Introduction to the drug
3.1. Chemistry
Tisagenlecleucel is an adoptive cell transfer therapy composed of chimeric antigen receptor-modified (CAR) T cells targeting CD19. To generate the drug, T cells are first collected from the patient, modified ex vivo using a lentiviral vector, expanded in the laboratory, and then reinfused into the patient. Specifically, the T-lymphocytes are engineered to express a murine single-chain fragment variable region (scFv) domain linked to the CD3-zeta signaling domain of the T-cell receptor (TCR) as well as a costimulatory domain, CD137 (4–1BB), with spacer and transmembrane domains derived from human CD8-α [9,34,35]. The antigen recognition sequence encoded by the scFv domain recognizes and binds to CD19, a target selected for its frequency of expression in B-cell leukemia, and whose cell-surface protein expression is limited to B cells and their precursors [36-38]. The costimulatory domain potentiates the cells, and is critical for their expansion and in vivo persistence [34,39,40]. The CD3-zeta domain is essential for initiating T-cell activation against tumor targets [9]. Combining the effector function of T lymphocytes with the ability of the scFv to recognize CD19 allows high binding specificity in a non-MHC restricted manner [41]. Other CD19-directed CARs have utilized a CD28 costimulatory domain with success; however, preclinical data suggest that the use of CD137 improves persistence potentially by ameliorating T-cell exhaustion [34,42].
3.2. Pharmacodynamics
Tisagenlecleucel is a targeted immune-modulating therapy that functions by binding a specific antigen present on malignant cells and activating the immune system to target and destroy tumor cells. Once manufactured, the transduced, autologous T-lymphocytes are reinfused and the chimeric antigen receptor binds to the CD19 protein on the surface of tumor cells. This interaction of tisagenlecleucel cells and CD19 results in the formation of immune synapses and initiates direct cytolytic tumor cell killing by the activation of the natural T-cell signaling pathways to produce and release cytokines. This signal simultaneously promotes cell expansion and differentiation through the costimulatory domain [43], thus resulting in persistent, selective toxicity against CD19-expressing cells. In both pediatric and adult patients, greater cellular expansion was seen in responding patients, compared to non-responders, consistent with the cytolytic mechanism of action: increased tisagenlecleucel activation against target cells enhances effector T-cell response and stimulates additional proliferation [40].
3.3. Pharmacokinetics and metabolism
Dosing for tisagenlecleucel depends on body weight, with a targeted cell dose for patients over 50 kg of 1.0–2.5 × 108 transduced viable T cells, and 2.0–5.0 × 106 cells for those 50 kg or less, with acceptable cell dose ranges of 0.2–2.5 × 106 and 0.1–2.5 × 108, respectively [35]. At these doses, no relationship between dose and cellular expansion has been observed [35]. The cellular kinetics of tisagenlecleucel differ from the pharmacokinetics of conventional therapies, for as a ‘living’ drug, its activity is mediated by dose administered as well as in vivo proliferation of the cells, followed by a persistence of the cells that is measurable from months to years [40,44]. Data from the most recently completed Phase-II trial demonstrated a median duration in blood of 168 days, with documented persistence as long as 20 months [35].
Tisagenlecleucel is administered intravenously and is therefore immediately bioavailable. Directly following infusion, there is a transient decline from peak infusion levels, attributed to distribution to the bone marrow, central nervous system (CNS), and other tissues, followed by a rapid expansion [40]. In responding patients, blood and marrow morphologic leukemia is typically cleared within 28 days [35,45,46]. Responders have a shorter median time to maximum expansion (10 days) and demonstrate more robust cellular expansion than non-responders [35]. Tisagenlecleucel demonstrates persistent distribution to the bone marrow with the tisagenlecleucel transgene reported at concentrations approximately half that noted in peripheral blood at Day 28, and at levels approximately 60–70% seen in peripheral blood at Months 3 and 6 [40]. At the same time points, tisagenlecleucel cells are also detectable in cerebrospinal fluid [40], likely a contributing factor to tisagenlecleucel’s efficacy in CNS disease [35,45-47].
4. Clinical efficacy
4.1. Phase-I studies
The CART19 trial, a Phase-I/IIa single-arm, single-center, open-label study (ClinicalTrials.gov NCT01626495 and NCT01029366), was the first trial to determine the safety, efficacy and cellular kinetics of tisagenlecleucel [46]. Thirty patients, 25 children and 5 adults, with relapsed or refractory CD19+ leukemia were studied. Eighteen patients had previously undergone allogeneic HSCT. The overall response rate, defined as either CR or CR with incomplete hematologic recovery, was 90% at one-month post-infusion. Nineteen patients (63%) demonstrated persistent remission at time of publication, with a median follow-up time of 7 months (range 1–24). Updated results focused on the pediatric cohort (n = 59) published in 2016 demonstrated a 93% overall response, with MRD-negative state achieved in 88% [45]. Relapse-free survival was 76% at 6 months, 55% at 12 months and overall survival was 79% at 12 months. Six patients experienced early relapse: in three patients relapse occurred following the early loss of tisagenlecleucel and in three patients after early B-cell reconstitution. Later relapses were associated with either loss of tisagenlecleucel persistence or CD19-negative escape variants [44,45].
4.2. Phase-II studies
The FDA approval of tisagenleclecuel was based on results of the ELIANA study (ClinicalTrials.gov NCT02435849). This is a single-cohort, multicenter, global study to test the safety and efficacy of tisagenlecleucel for children and young adults with relapsed or refractory B-cell ALL [35]. The primary outcome analysis included 92 patients, 17 of whom were excluded due to tisagenlecleucel product-related issues, death or other adverse events that precluded tisagenlecleucel infusion. The 75 who received tisagenlecleucel had undergone a median of 3 previous therapies and had a median bone marrow blast percentage of 74%. A majority of them (61%) had previously undergone allogeneic HSCT. Ninety-six percent of those who received tisagenlecleucel received lymphodepleting chemotherapy in preparation; the three who did not undergo conditioning chemotherapy were already leukopenic. The overall response rate for patients who received tisagenlecleucel was 81% (95% CI, 71–89) at three months. Overall survival was 90% (95% CI, 81–95) at 6 months, and 76% (95% CI, 63–86) at 12 months. For responders, relapse-free survival was 80% (95% CI 65–89) at 6 months, and 59% (95% CI, 41–73) at 12 months. Relapse was largely driven by CD19-negative escape variants: at relapse, a single patient was CD19+, 15 patients were CD19−, and 6 patients had unknown CD19 status. Event-free survival was 73% (95% CI, 60–82) at 6 months, and 50% (95% CI, 35–64) at 12 months. Eight patients underwent allogeneic HSCT while in tisagenlecleucel-induced remission, including two patients who were MRD + and two patients with evidence of early B-cell reconstitution.
4.3. Summary table of Phase-I and -II trial results
See Table 1.
Table 1.
Summary table of Phase-I and -II trial results for tisagenlecleucel and other drugs labeled for the same indication.
| Clinical trials number |
Patient population |
Preparatory chemotherapy |
Phase | n (ITT/ received drug) |
Complete response* rate |
6 month/ 12 month relapse free survival |
Median relapse free survival (months) |
6 month/ 12 month overall survival |
Median overall survival (months) |
Patients with HSCT after treatment (n) |
Reference number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tisagenlecleucel | |||||||||||
|
NCT01626495 NCT01029366 |
|
Physician discretion | 1/2a | 30/30 |
|
67%/NR | Not reached | 78%/NR | Not reached | 3 | [46] |
|
59/59 |
|
76%/55% | NR | NR/79% | NR | 5 | [45] | |||
| NCT02435849 |
|
Fludarabine/Cyclophosphomide | 2 | 92/75 |
|
73%/50% | 19.1 | 90%/76% | Not reached | 8 | [35] |
| Yescarta | |||||||||||
|
NCT02614066 NCT02625480 |
|
Fludarabine/Cyclophosphomide | 1/2 | 6/5 |
|
NR | NR | NR | NR | NR | [60] |
| Other CD19 CARs | |||||||||||
| NCT02028455 |
|
Fludarabine/Cyclophosphomide | 1/2 | 45/43 |
|
NR/51% | ~ 12 | NR/70% | ~ 24 | 11 | [66] |
| NCT01593696 |
|
Fludarabine/Cyclophosphomide | 1 | 21/21 |
|
NR | NR | NR | 9.7 | 10 | [61] |
| NCT01593696 |
|
Varied | 1 | 51/51 |
|
NR | 18 (for MRD negative responders) | NR |
|
21 | [82] |
| NCT01865617 |
|
Cyclophosphamide ± fludarabine | 1/2 | 32/30 |
|
NR |
|
NR | NR | NR | [56] |
| Blinatumomab | |||||||||||
| Compassionateuse |
|
n/a | n/a | −/9 |
|
NR | ~ 8.3 | NR | ~ 13.3 | 5 | [32] |
| NCT01466179 |
|
n/a | 2 | 189/189 |
|
~ 50%/~ 30% | 5.9 | ~ 50%/~ 28% | 6.1 | 32 | [29] |
| NCT02013167 |
|
n/a | 3 | 271/267 |
|
31%/NR | 7.3 | 54%/~ 12% | 7.7 | 65 | [30] |
| NCT01471782 |
|
n/a | 1/2 | 93/70 |
|
42%/NR | 4.4 | NR | 7.5 | 24 | [31] |
| Inotuzumab | |||||||||||
| NCT01564784 |
|
n/a | 3 | 164/109 |
|
NR | 4.6 | NR | 7.7 | 45 | [33] |
| Clofarabine | |||||||||||
| NCT00042341 |
|
n/a | 2 | 62/61 |
|
NR | 2.4 (among patients with at least partial response) | NR | 3.3 | 9 | [19] |
| Vincristine | |||||||||||
| NCT00495079 |
R/R ALL |
n/a | 2 | 65/65 |
|
35%/8% | 5.8 | NR | 4.6 | 12 | [16] |
ITT, intention to treat; HSCT, hematopoietic stem cell transplant; R/R ALL, relapsed or refractory acute lymphoblastic leukemia; MRD, minimal residual disease; NR, not reported; NHL, non-Hodgkin lymphoma.
Complete response is defined as clinical remission and clinical remission with incomplete marrow recovery unless otherwise noted; results based on patients infused (ITT data not always available).
4.4. Phase-III studies
There are no Phase-III study results available.
5. Tisagenlecleucel for other B-cell malignancies
Tisagenlecleucel has also been tested for adults with B-cell non-Hodgkin lymphoma NHL and has recently been approved by the FDA for the treatment of relapsed or refractory B-cell lymphomas including diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma and DLBCL arising from follicular lymphoma. This approval was based on the results from the global single-arm, Phase-II JULIET trial (NCT02445248). In this trial, 32% of patients showed CR at 3 months (n = 81), and nearly all of those who responded to the therapy at 3 months remained relapse-free at 6 months [48]. A similar single institution trial reported complete remission rates of 43% (n = 14) for DLBCL and 71% (n = 14) in patients with follicular lymphoma [49]. Tisagenlecleucel has also been tested for adults with chronic lymphocytic leukemia (CLL). Results were first reported as a case report in 2011, citing excellent tumor response [50]. A later pilot study confirmed tisagenlecleucel’s clinical efficacy for CLL, with an overall response rate of 57% and a 29% CR rate [51]
6. Other anti-CD19 CARs
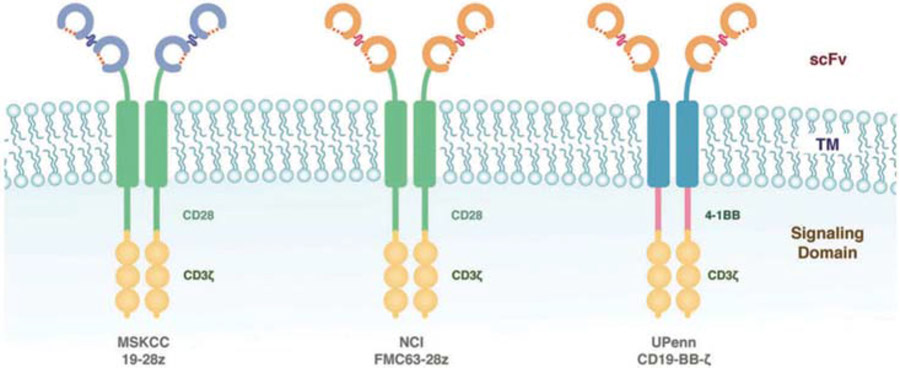
Other CD19-targeted CAR constructs have also demonstrated promising results with high anti-tumor efficacy in children and adults with relapsed B-cell ALL, CLL, and NHL. The key distinguishing difference between CAR designs is the costimulatory domain (Figure 1). Tisagenlecleucel is constructed using a CD137 (4-1BB) domain, which is thought to account for the improved persistence of this CAR in comparison to CARs employing CD28-based costimulation [42]. Other products under investigation also include differences in T-cell transduction methods––tisagenlecleucel is transduced using a lentivirus, other constructs use γ-retroviruses––as well as infused CAR-T-cell doses, and antecedent lymphodepleting chemotherapy regimens [52]. The comparison of early phase trial results across these immunotherapy products, however, is difficult. The eligibility criteria, including disease type, previous treatment exposures, and patient age, are not the same for all trials. Similarly, there is no standard outcome reporting.
Figure 1.
Schematic diagram of CAR-T-cell products used to treat ALL. Tisagenlecleucel utilizes an scFv domain linked to the CD3-zeta signaling domain and a CD137 (4-1BB) costimulatory domain. Other CARs in development or in clinical trials utilize a CD28 costimulatory domain.*CAR developed at Memorial Sloan-Kettering (MSKCC), CAR developed at National Cancer Institute (NCI), and tisagenlecleucel (UPenn); CAR, Chimeric Antigen Receptor; ALL, acute Lymphoblastic leukemia. Reproduced from Davila et al. How do CARs work? Early insights from recent clinical studies targeting CD19. OncoImmunology 2012 1:9, 1577–83.
Similar to tisagenlecleucel, JCAR017, developed through the Fred Hutchinson Cancer Center, incorporates a 4-1BB costimulatory domain, but instead undergoes a separate expansion and infusion of CD4 and CD8T cell subsets based on the hypothesis that a defined CD4:CD8T cell composition may allow for increased potency from a lower cell dose [53]. Early results demonstrate a CR rate similar to tisagenlecleucel in children and young adults with B-ALL (93% among those who received a CAR T cell product), promising overall survival [54], and strong results in adult NHL/CLL [55,56]. Studies in children with this product are ongoing (ClinicalTrials.gov NCT01683279).
Axicabtagene ciloleucel (Yescarta™, Kite Pharmaceuticals), a CD19-targeted CAR construct that employs a CD28 costimulatory domain, was recently approved by the FDA for the treatment of specific types of relapsed or refractory NHL in adults [57-60]. Phase-I trials in children with B-ALL demonstrated a CR rate of 67% and a 10-month overall survival of 51.6% [61]. A multicenter Phase-II trial is ongoing (ClinicalTrials.gov NCT02625480). Investigators at Memorial Sloan-Kettering Cancer Center have also engineered a CD19-targeted CAR using the CD28 costimulatory domain that has been trialed in adult ALL, demonstrating a CR rate of 82% and a 6-month overall survival of 65% [62,63].
Identified mechanisms of leukemia relapse following chemotherapy treatment include evolution from an ancestral clone and intrinsic chemotherapeutic drug resistance, as well as acquired resistance with the development of new somatic alterations conferring chemo-resistance or altering signaling different signaling pathways. Rarely, ‘relapse’ actually represents a new biologically distinct leukemia; this is more commonly seen in T-ALL as compared with B-ALL. The overwhelming majority of relapses arise from the clone present at diagnosis [64]. Similar mechanisms have been identified for relapse following CD19-directed therapy, the majority of which are CD19-negative escape variants or loss of CAR-T-cell persistence [46,61,65,66]. These relapses have been observed in ALL patients treated with different CART19 products and likely are independent of the CAR construct, expansion methods, or clinical protocol [65]. Several possible mechanisms have been described to explain CD19-loss escapes, including pre-existing CD19-negative subpopulations existent in the leukemia bulk [44] (intrinsic resistance), truncated CD19 protein that does not trigger CD19-directed lysis [67] (acquired resistance), and, in a small proportion of cases, myeloid lineage switch [68]. The driving mechanisms of each are still not understood well enough to target directly, nor is there adequate knowledge of who is at increased risk for relapse following CD19-directed therapy. Lineage switch appears to be more common in KMT2A-R (MLL-R) B-ALL than other biologic subtypes, but more studies are needed.
Initial methods to surmount relapse following CD19 directed therapy have included multiple infusions of CAR T cells to overcome loss of persistence, the development of alternate targets, including CD22-directed CAR T cells (ClinicalTrials.gov NCT02315612) [69], a humanized construct designed to overcome the potential of murine-based of immune-mediated rejection [70], and the use of immune checkpoint blockade to restore or enhance CAR-T-cell persistence [71,72]. Bi-specific CAR T targets and universal CAR T cells, discussed more in depth below, are also being explored.
7. Post-marketing surveillance
There have not been head-to-head randomized trials comparing short- and long-term toxicity between tisagenlecleucel and standard therapy, but specific side effects have emerged related to the immunomodulatory and targeted effects of tisagenlecleucel as well as other CAR-T-cell therapies: cytokine release syndrome (CRS), neurologic events, hypogammaglobinemia, and acute infusion reactions. Nevertheless, the overall frequency and severity of toxicities is lower with CAR-T cells than those reported with multi-agent chemotherapy regimens. Similar toxicities can also occur with BiTEs.
The most common and potentially severe toxicity associated with tisagenlecleucel is CRS. CRS is a systemic inflammatory response syndrome resulting from activated T-cell proliferation and release of high levels of inflammatory cytokines which can lead to profound shock and multiple organ dysfunction syndrome [37,38,73]. Biologically, CRS mirrors hematophagoctyic lymphohistiocytosis (HLH) and macrophage activation syndrome (MAS), with overlapping clinical, laboratory, and cytokine profiles [74]. CRS symptoms include constitutional complaints (high fevers, rigors, fatigue, myalgia and arthralgia), gastrointestinal distress (anorexia, nausea, vomiting), neurologic symptoms (encephalopathy, headache), respiratory complaints (dyspnea, tachypnea, hypoxia), elevated laboratory markers of inflammation (ferritin, LDH, and CRP), and organ system dysfunction as manifested by hyperbilirubinemia, transaminitis, kidney injury, transient left ventricular dysfunction, coagulopathy and hypotension. Marked proliferation of tisagenlecleucel cells [35,44,46] and high disease burden at the time of treatment are the primary identified risk factors for a severe CRS reaction [46,74]. The positive predictive value (PPV) of high disease burden alone is poor; however, low disease burden does have a strong negative predictive value (NPV) for the development of severe CRS [74]. In severe CRS, hypercytokinemia results from both activated T-cells and aberrantly activated macrophages. This is evidenced by a cytokine prolife that includes elevated interferon gamma (INF-γ interleukein 10 (IL-10), and IL-6. While high levels of IL-6 could be expected from activated T-cells alone, marked elevations of INF-γ and IL-10 would not be expected. Recent work also attempted to define biomarkers that may predict severe CRS, eg laboratory tests that can be sent after infusion with CAR T cells but before a patient becomes critically ill that predict which patients will become critically ill. Examples, include a combination of IFN-γ, IL13, and MIP1-α as well as disease measuring disease burden prior to infusion and IL-10 levels 1–2 days after infusion. These predictive models need to be validated prospectively.
CRS was reported in 88% patients in the CART19 trial, and 77% of those enrolled in the ELIANA trial [35,44-46]. The median time to onset after infusion was three days, and symptoms lasted an average of eight days [35]. Across both trials, a total of 51 patients (49%) were admitted to the intensive care unit for management of CRS. Supportive care treatment for these cases included respiratory and hemodynamic support, including 10 cases necessitating mechanical ventilation [35] and seven requiring dialysis [35].
Typically, initial management of CRS includes supportive care measures with oxygen, judicious use of intravenous fluids, low-dose vasopressors and anti-pyretics [35]. Patients who develop severe CRS are additionally treated with cytokine blockade. The concept of cytokine blockade after treatment with CAR T cells was pioneered at CHOP/UPENN after the first pediatric patient treated with tisagenlecleucel became critically ill with CRS. There was concern she might not survive and was treated with tocilizumab, a humanized monoclonal anti-body against IL-6R based on her high serum IL-6 levels. Following tocilizumab administration, she experienced a rapid improvement in CRS. Tocilizumab, now the cornerstone of cytokine-based therapy following treatment with tisagenlecleucel, is effective at mitigating CRS without decreasing the efficacy of the drug [35,37,44,52,61,75] or abrogating cellular expansion [40]. As such, it was recently FDA-approved for the treatment of severe CRS after CAR T-cell therapy. Siltuximab, an IL-6 antagonist, has also been used in the management of CRS. More data are needed to determine if there is a superior agent. Corticosteroids (including methylprednisolone, hydrocortisone, dexamethasone) have been used in the event of tocilizumab-refractory CRS [35], but are generally reserved for use only following tocilizumab failure because of concerns of impact on tisagenlecleucel’s efficacy, particularly if administered shortly after CAR T-cell infusion. In the ELIANA and CART19 trials, 37 patients received tocilizumab [35,46], including four patients who received a second dose for recrudescence of CRS after transient improvement with the first dose [46]. Six patients also received short courses of corticosteroids for management of CRS [46]. There is evidence to suggest that prophylactic intervention with immunomodulation can decrease rates of severe CRS [76] and there is an ongoing trial (Clinicaltrials.gov NCT02906371) to evaluate the efficacy of using early administration of tocilizumab to prevent tisagenlecleucel-associated CRS safety events in pediatric patients.
Neurologic events were also common, with rates between 40 and 44% in both Phase-I and -II trials. Reported symptoms ranged from delirium to global encephalopathy; including aphasia, confusion, hallucination, tremor, agitation, and seizure [35,46]. CAR T-related neurotoxicity, recently termed CAR T-cell-related encephalopathy syndrome (CRES) [77], is an off-target toxicity that may result from excessive T-cell activation, but is distinct from CRS. The biology of neurotoxicity is incompletely understood. Although it is known that tisagenlecleucel crosses the blood brain barrier and can persist in the CNS for months, presence of tisagenlecleucel in the cerebrospinal fluid has not been found to correlate with severity of CRES [40]In addition, CRES does not appear to be readily reversed or ameliorated by IL-6 receptor blockade, suggesting an alternative pathophysiology [78]. Of note, most cytokine blockers including tocilizumab are monoclonal antibodies and do not cross the blood brain barrier. Current work proposes that T-cell activation after infusion causes CNS endothelial cell activation leading to coagulopathy and endothelial cell permeability. The resultant blood brain barrier breakdown allows passage of inflammatory cytokines and T cells into the CNS, further activating endothelial cells and accelerates the pathophysiology [79]. In Phase-I and -II trials, the majority of CRES occurred during, or shortly after, CRS [35]. Severity of CRES correlated with severity of CRS in the ELIANA trial [35], but did not appear to correlate in the CART19 trial [46]. In contrast to trials with other CAR products that use alternative co-stimulatory domains [80], no grade 5 neurologic events or cerebral edema were reported in either tisagenlecleucel trial [35,46]. The majority of patients with tisagenlecleucel-related CRES experienced full recovery from neurotoxicity with supportive management alone.
An expected result of ‘on-target’ effect of tisagenlecleucel in patients with a sustained tumor response is depletion of B cells with resulting hypogammaglobinemia [38,39]. In fact, B-cell monitoring can be used as a marker for tisagenlecleucel’s persistence, as early B-cell recovery, marked by B-cell return prior to three months after infusion, is associated with risk of relapse [40,46]. Hypogammaglobinemia is typically managed with immunoglobulin replacement therapy based on age-specific guidelines and local practice [35].
Other severe adverse events that occurred in at least 5% of patients included cytopenias, electrolyte and liver function test abnormalities, fever, anorexia, hypotension, hypoxia, pulmonary edema, acute kidney injury, and fluid overload [35]. Coagulopathy has been noted as a late effect, occurring in many cases following the resolution of CRS, with elevated prothrombin and partial-thromboplastin times, as well as hypofibrinogenemia in patients who had experienced severe CRS. Similar to HLH, the degree of hypofibrinogenemia is disproportionally worse compared to the changes seen in other coagulation factors. Clinical bleeding was rare [44,46], but monitoring of laboratory values and avoidance of anticoagulation, if possible, is important. Acute infusion reactions also occur, including fever, chills, and nausea. These are mitigated by the use of pre-medication with an antipyretic, diphenhydramine and an H1 anti-histamine. Steroids are avoided except in the case of life-threatening reaction, as administration of steroids may have an adverse effect on the cell function and expansion.
The long-term side effects are unknown and more data are needed. It is anticipated that the side effect profile will be less than that seen with intensive chemotherapy, radiation, or transplant.
7.1. Table summarizing safety outcomes in clinical trials
See Table 2.
Table 2.
Summary of safety outcomes in clinical trials for tisagenlecleucel and other drugs labeled for the same indication.
| Treatment related devent |
CRS/Immune event |
Neurologic event |
Other common events |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | Any grade | Grade 3–5 | Any grade | Grade 3–5 | Any grade | Grade 3–5 | Any grade | Grade 3–5 | References |
| – * | – | 30 (100%) | 8 (27%) | 13 (43%) | – | – | – | [46] | |
| Tisagenlecleucel | – | – | 52 (88%) | 16 (27%) | – | – | – | – | [45] |
| 71 (95%) | 55 (73%) | 58 (77%) | 35 (47%) | 30 (40%) | 10 (13%) |
|
|
[35] | |
| Other CD19-directed CART cells | |||||||||
| MSKCC | – | – | – | 9 (56%) | – | 1 (6%) | – |
|
[62] |
| FHCRC | – | – | 25 (83%) | 7 (23%) | 15 (50%) | 15 (50%) | – | – | [56] |
| NCI | – | – | – | 7 (13%) | 5 (9%) | 3 (6%) | – | – | [82] |
| 188 (99%) | 155 (82%) | 3 (2%) | 0 | 98 (52%) | 24 (13%) |
|
|
[29] | |
| Blinatumomab | 263 (99%) | 231 (87%) | – | 13 (5%) | – | 25 (9%) | – |
|
[30] |
| Inotuzumab | 67 (48%) | 64 (46%) | – | – | – | – |
|
|
[33] |
| Clofarabine | – | – | – | – | – | 8 (13%) | – |
|
[19] |
| Vincristine | 53 (82%) | 37 (57%) | – | – | 41 (63%) | 13 (20%) |
|
|
[16] |
CRS, cytokine release syndrome; CAR, chimeric antigen receptor; MSKCC, Memorial Sloan Kettering Cancer Center; FHCRC, Fred Hutchinson Cancer Research Center; NCI, National Cancer Institute; GI, gastrointestinal.
Data on adverse event not specifically included in publication.
8. Regulatory affairs
The FDA approved tisagenlecleucel in August 2017 for the treatment of patients up to age 25 years with B-cell precursor ALL that is refractory or in second or later relapse. This was the first FDA approval granted to a chimeric antigen receptor T-cell immunotherapy [45]. Tisagenlecleucel has subsequently been approved for the treatment of relapsed or refractory large B-cell lymphomas in adults. Axicabtagene ciloleucel, another CD19-targeted CAR T-cell therapy was later granted FDA approval in October 2017 for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy [59]. Neither agent has yet been approved by the EMA, although both have been submitted for consideration. Tisagenlecleucel is currently under review by the EMA for two labeling indications: for the treatment of children and young adults with relapsed or refractory B-cell ALL and for adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for autologous stem cell transplant.
9. Conclusion
In both Phase-I and -II trials, tisagenlecleucel has demonstrated high remission rates and more durable remissions compared to other agents used in the standard care of pediatric and young adult patients with multiply-relapsed or refractory B-cell ALL [35,38,44-46]. Treatment with tisagenlecleucel has a risk of serious toxicity, including CRS and severe neurologic symptoms which can require ICU-level care, but can be mitigated with cytokine blockade and supportive care measures [35,37,73,81]. Other CAR-T-cell therapies are currently under investigation, but do not yet have enough data to support use outside of clinical trials.
9.1. Expert commentary
Tisagenlecleucel is a novel therapeutic for the treatment of pediatric and young adult ALL. Results from early phase trials demonstrate its potent and lasting antitumor effects, providing patients a durable remission despite, in some cases, multiple failed prior regimens. This is an exciting move forward in the treatment of multiply-relapsed or refractory ALL.
Future trials will undoubtedly focus on the use of tisagenlecleucel in an upfront setting. But an equally important advance in the field should be optimizing tisagenlecleucel’s use in the context of other available therapies. It is not yet understood if the type of conditioning regimen matters: lymphodepleting regimens in both the ELIANA and CART19 trials were largely physician-choice and did not appear to impact outcomes; whereas investigators using other CARs have found difference in outcomes based on pre-infusion regimens [61,82]. In addition, it will be important to evaluate if the upfront concurrent or sequential use of other targeted agents, such as CD22-directed inotuzumab, will be helpful in surmounting CD19-negative escape relapses.
In addition, the question of whether consolidation with allogeneic HSCT is required to maintain the remission induced by CAR-T cells remains largely unanswered. Early B-cell recovery may be a harbinger of relapse, suggesting that this is one criteria that could recommend HSCT after tisagenlecleucel; however, other patients have remained in a durable molecular remission despite undetectable circulating CAR T cells [35,46]. In contrast, CD-19 negative relapses occur in the presence of persisting CAR T cells. Long-term follow-up of children who have received a different CAR-T construct demonstrated that relapse was significantly more common in patients who did not undergo HSCT following CAR therapy (85.7% versus 9.5%, p = .0001) [82]. However, the ability to make inferences on the role of HSCT after tisagenlecleucel specifically is difficult. More than half of the patients in the ELIANA and CART19 trials had undergone HSCT prior to trial enrollment. Eleven patients underwent HSCT after treatment with tisagenlecleucel (of 105 patients total), 9 while their disease was still in a molecular remission [35,46]. Although historically HSCT has been the only curative option for patients with multiply relapsed or refractory ALL, this therapy carries significant rates of long-term morbidity and mortality, including infertility, chronic graft-versus-host disease, neurologic impairment and secondary malignancies. Moreover, in patients who receive CAR-T-cell therapy as a bridge to transplant, the conditioning used for HSCT eliminates the remaining circulating CAR T-cells that were effective in inducing the remission, which therefore may increase the risk of relapse. Because tisagenlecleucel has been effective in maintaining long-term durable remissions and the late effects after CAR-T-cell therapy are expected to be far less morbid than HSCT, tisagenlecleucel should be considered definitive therapy for a subset of patients. Certainly included in this subset are patients who relapse after HSCT: it does appear both safe and feasible for patients to receive donor-derived CAR T-cell therapy for relapse after HSCT. The optimal therapeutic window for infusion of CAR T-cell therapy after HSCT and the efficacy of this therapy for the prevention of relapse once molecular relapse is detected are additional areas for future study [83]. Additional research efforts should be directed toward understanding the clinical phenotype, genetic classification of the leukemia, and the impact of MHC allele characteristics that predict a durable response from CAR T-cell therapy alone. In the absence of randomized comparative studies of immunotherapy versus transplant, other patients for whom tisagenlecleucel could be considered definitive therapy include those with disease biology associated with poor prognosis or persistent MRD positivity despite intensive chemotherapy and patients with significant medical comorbidities as the outcomes for these patients are dismal with HSCT. As our understanding of which patients will benefit from HSCT consolidation therapy after tisagenlecleucel evolves, this decision should be made on an individual basis that incorporates the patient’s known risk factors and previous therapies, as well as patient and family preference.
It is essential that future trials of all CD19-directed CAR therapies standardize outcome and toxicity reporting, to allow for direct comparison across products despite lack of randomized controlled trials [52]. Details regarding the timing and MRD status at the time of HSCT should be explicit for those patients who undergo transplant following receipt of tisagenlecleucel. Specifics regarding those patients who fail to respond––because of cellular expansion failure either prior to, or after, infusion––should be made easily available. As important as consistent outcome measures, attention must also be paid to standardizing toxicity reporting. Tisagenlecleucel and other CD19-directed CAR therapies come with significant attendant risks. In the absence of head-to-head trials, trial results should include cross-comparable scales for the most commonly encountered toxicities: namely CRS and adverse neurologic events. Although CRS has been previously graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, this grading schema is inadequate for cellular therapy-associated CRS [84,85]. In response, individual centers conducting immunotherapy trials have created their own scales for characterizing the severity of CRS [62,77,86,87]. The University of Pennsylvania, based on their experience with tisagenlecleucel specifically, has published a standard CRS grading scale that depends only on accessible clinical features without a reliance on quantitation of supportive care or patient location, applies to early-onset and delayed-onset CRS, and distinguishes between mild, moderate, severe, and life-threatening CRS associated with T-cell therapies [84]. Utilization of this scale––or a consensus agreement of an alternate grading schema––should be adopted across CAR therapy trials.
9.2. Five-year view
Strategies to enhance the efficacy of CD19-targeted CARs are currently under development. Dual-specific CD19/CD22 and CD19/CD123 directed CAR T cells, a strategy designed to help prevent antigen loss and avoid relapse via CD19-negative escape variants, are currently in development or under investigation in both adults and children (ClinicalTrials.gov NCT03330691; NCT03241940) [88]. In addition, universal CARs––products developed from healthy donor T cells that have been gene edited to disrupt expression of endogenous TCRs in order to avoid alloreactivity––have been used in two pediatric patients with ALL and successfully induced remission [89]. Although one child had prolonged B-cell aplasia and the investigators recommend these products only be used as a bridge to transplant based on the theoretical risk of genotoxicity from TALEN-induced translocations. More work is needed, however, to minimize the risk of rejection and graft-versus-host-disease associated with these agents. Finally, to abrogate long-term toxicities associated with CAR-T-cell therapy, research is ongoing on the incorporation of a suicide gene––genetically-encoded molecules that allow selective ablation of gene-modified cells responsible for unwanted or off-target effects [90,91]. (Investigation of tisagenlecleucel’s efficacy in other CD19+ malignancies will continue: there have already been promising results when used in the treatment of adults with diffuse large B-cell lymphoma [92]. We can similarly expect to see further development and refinement of chimeric antigen receptor T-cell products for other malignancies [93-96].
In the future, adoptive T-cell therapy may become the first-line treatment for relapsed or refractory B-ALL given the remission and overall survival rates seen in Phase-I and-II trials. As experience with this therapy grows, it may be possible to transition its use to the upfront treatment of primary B-cell malignancies. Management of toxicity will improve, as ongoing trials help to establish who is at greatest risk and how to best reverse or prevent it [97]. Comparative effectiveness trials with standardized outcome and toxicity reporting will enhance our understanding of how to best apply the CAR-T-cell products now available, and combination regimens that optimize conditioning regimens and minimize CD19-escape driven relapses will be developed. Tisagenleclucel’s approval by the FDA ushers in a new treatment paradigm for ALL. We are certain that this will improve the survival of children and young adults with ALL and are optimistic that these successes will translate to the treatment of other malignancies.
Key issues.
Tisagenlecleucel is a highly effective therapy for pediatric and young adult patients with refractory or recurrent relapsed (r/r) B-cell acute lymphoblastic leukemia (B-ALL).
Tisagenlecleucel was the first FDA-approved cellular therapy
Tisagenlecleucel is a CD19-directed chimeric antigen recep-tor-modified (CAR) T-cell therapy that is constructed using a lentivirus and employs a 4–1BB (CD137) costimulatory domain.
No other agent currently labeled for r/r B-ALL–including clo- farabine, blinatumomab, inotuzumab, or liposomal vincris-tine–provides similar disease-free or overall survival rates.
Other CD19-directed chimeric antigen receptor T-cell thera-pies have been developed and report promising results, but there may be differences in the duration of response across these agents.
The most significant toxicity associated with tisagenlecleu- cel is cytokine release syndrome which can be managed with supportive care and cytokine blockade, specifically with tocilizumab, an IL6-receptor binding antibody. Other toxicities include neurologic toxicity and hypogammaglobulinemia.
Future research should focus on understanding how to incorporate tisagenlecleucel with current treatment modal-ities, on optimizing toxicity management, and on develop-ing analogous CAR T cells with alternative targets.
Declaration of interest
A Barz Leahy reports being the recipient of a National Institute of Health grant (T32HD060550-07). CW Elgarten reports receiving a National Institutes of Health Clinical Pharmacoepidemiology grant (T31- GM075766). SA Grupp reports receiving grants and personal fees from Novartis, as well as personal fees from Adaptimmune. SL Maude is a St. Baldrick’s Foundation Scholar and reports receiving personal fees from Novartis. DT Teachey reports receiving institutional funds from Novartis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose. Novartis provided a scientific accuracy review at the request of the journal editor.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.National Cancer Institute. SEER cancer stat facts: acute lymphocytic leukemia [Internet]. Bethesda, MD. cited 2018 Jan Available from: http://seer.cancer.gov/statfacts/html/alyl.html [Google Scholar]
- 2.Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–2511. [DOI] [PubMed] [Google Scholar]
- 3.Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012. Jan 5;119:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia?. Hematol Am Soc Hematol Educ Program. 2012; 2012:129–136. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez A, Silverman LB. Acute lymphoblastic leukemia. Nathan and oski’s hematology and oncology of infancy and childhood. Eighth. Philadelphia: Elsevier Health Sciences; 2015. p. 1527–1555. [Google Scholar]
- 6.Nguyen K, Devidas M, Cheng S-C, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrappe M, Hunger SP, Pui C-H, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012. Apr 12;366:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierro J, Hogan LE, Bhatla T, et al. New targeted therapies for relapsed pediatric acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2017. Jul 13;17:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KYMRIAH™ (tisagenlecleucel) [Package Insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp., 2017. Aug 30. [Google Scholar]
- 10.Pui C-H, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013. Jul;50:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. The Lancet. 2010;376:2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locatelli F, Schrappe M, Bernardo ME, et al. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012. Oct 4;120:2807–2816. [DOI] [PubMed] [Google Scholar]
- 13.Bhojwani D, Pui C-H. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013. May;14:e205–17. [DOI] [PubMed] [Google Scholar]
- 14.Sun W, Orgel E, Malvar J, et al. Treatment-related adverse events associated with a modified UK ALLR3 induction chemotherapy backbone for childhood relapsed/refractory acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016. Jul 20;63:1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Marqibo, vincristine liposome injection, FDA label. 2012 [Google Scholar]
- 16.O’Brien S, Schiller G, Lister J, et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol. 2013. Feb 20;31:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelken B, Cave H, Leverger G, et al. A phase I study of clofarabine with multiagent chemotherapy in childhood high risk relapse of acute lymphoblastic leukemia (VANDEVOL study of the french sfce acute leukemia committee). Pediatr Blood Cancer. 2016;63:270–275. [DOI] [PubMed] [Google Scholar]
- 18.Pathak P, Hess R, Weiss MA. Liposomal vincristine for relapsed or refractory Ph-negative acute lymphoblastic leukemia: a review of literature. Ther Adv Hematol. 2014. Feb;5:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeha S, Gaynon PS, Razzouk BI, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006. Apr 20;24:1917–1923. [DOI] [PubMed] [Google Scholar]
- 20.Hijiya N, Barry E, Arceci RJ. Clofarabine in pediatric acute leukemia: current findings and issues. Pediatr Blood Cancer. 2012. Sep;59:417–422. [DOI] [PubMed] [Google Scholar]
- 21.Pulsipher MA, Wayne AS, Schultz KR. New frontiers in pediatric Allo-SCT: novel approaches for children and adolescents with ALL. Bone Marrow Transplant. 2014. Oct;49:1259–1265. [DOI] [PubMed] [Google Scholar]
- 22.Oskarsson T, Söderhäll S, Arvidson J, et al. Treatment-related mortality in relapsed childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2017. Dec 12;127:e26909. [DOI] [PubMed] [Google Scholar]
- 23.Jabbour E, O’Brien S, Ravandi F, et al. Monoclonal antibodies in acute lymphoblastic leukemia. Blood. 2015. Jun 25;125:4010–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Drug Administration. Besponsa, FDA Label. 2017. Aug 17 [Google Scholar]
- 25.Food and Drug Administration. BLINCYTO® (blinatumomab), FDA label. 2017. Jul 12 [Google Scholar]
- 26.European Medicines Agency. BLINCYTO (blinatumomab), EMA Label. 2017. Sep [Google Scholar]
- 27.European Medicines Agency. Besponsa, INN-inotuzumab ozogamicin, EMA Label.Nov 20 2017. [Google Scholar]
- 28.Food and Drug Administration. BLINCYTO® (blinatumomab), FDA Label. 2018. Mar 29 [Google Scholar]
- 29.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med. 2017. Mar 2;376:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stackelberg Von A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016. Dec 20;34:4381–4389. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel P, Lang P, Zugmaier G, et al. Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica. Haematolo. 2014;99:1212–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl Med Mass Med Soc. 2016. Aug 25;375:740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018. Feb 1;378:439–448. •• Phase-I/II trial assessing the use of tisagenlecleucel in pediatric and young adult patients with relapsed or refractory B-ALL.
- 36.Sadelain M. CAR therapy: the CD19 paradigm. J Invest Am Soc Clin Investig. 2015. Sep;125:3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maude SL, Barrett D, Teachey DT, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. The Cancer Journal. 2014;20:119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maude SL, Teachey DT, Porter DL, et al. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015. Jun 25;125:4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011. Aug 10;3:95ra73–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017. Nov 23;130:2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011. Dec 21;480:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long AH, Haso WM, Shern JF, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu XV. KYMRIAH summary basis for regulatory action [Internet]. CBER Food Drug Admin. 2017. Aug:1–21. Available from:https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM577221.pdf [Google Scholar]
- 44.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl Med Mass Med Soc. 2013. Apr 18;368:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL (abstract). J Clin Oncol. 2018. Feb 21;34:3011. [Google Scholar]
- 46. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014. Oct 16;371:1507–1517. •• Phase-I trial assessing the use of tisagenlecleucel in pediatric and young adult patients with relapsed or refractory CD19-positive leukemia.
- 47.Rheingold SR, Chen L, Maude SL, et al. Efficient trafficking of chimeric antigen receptor (CAR)-modified t cells to csf and induction of durable CNS remissions in children with CNS/combined relapsed/refractory ALL. Blood. 2015;126:3769. [Google Scholar]
- 48.Leslie M. Value in using CAR T cells for DLBCL. Cancer Discov. 2018. Feb;8:131–132. [DOI] [PubMed] [Google Scholar]
- 49.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl Med Mass Med Soc. 2017. Dec 28;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter DL, Levine BL, Kalos M, et al. chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011. Aug 25;365:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porter DL, Hwang W-T, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015. Sep 2;7:303ra139–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016. Jun 30;127:3312–3320. • A comprehensive review of available anti-CD19 CAR therapies for hematologic malignancies.
- 53.Sommermeyer D, Hudecek M, Kosasih PL, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gardner R, Finney O, Leger K, et al. CD19CAR T cell products of defined CD4: CD8Composition and transgene expression show prolonged persistence and durable MRD-negative remission in pediatric and young adult B-cell ALL ∣. Blood. 2016;128:219. [Google Scholar]
- 55.Turtle CJ, Berger C, Sommermeyer D, et al. Anti-CD19 chimeric antigen receptor-modified T cell therapy for B cell non-hodgkin lymphoma and chronic lymphocytic leukemia: fludarabine and cyclophosphamide lymphodepletion improves in vivo expansion and persistence of CAR-T cells and clinical outcomes. [Abstract]. Blood. 2015;126: 184. [Google Scholar]
- 56.Turtle CJ, Hanafi L-A, Berger C, et al. CD19 CAR–T cells of defined CD4+: CD8+composition in adult B cell ALL patients. J Clin Investig. 2016. Jun 1;126:2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015. Feb 20;33:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neelapu S, Locke F, Bartlett N, et al. A phase 2 multicenter trial of KTE-C19 (anti-CD19 CAR T cells) in patients with chemorefractory primary mediastinal B-cell lymphoma (PMBCL) and transformed follicular lymphoma (TFL): interim results from ZUMA-1 ∣. Blood. 2016;128:998. [Google Scholar]
- 59.Kite Pharmaceuticals. YESCARTA (axicabtagene ciloleucel) [Package Insert]. 2017. Oct 19
- 60.Shah B, Huynh V, Sender LS, et al. High rates of minimal residual disease-negative (MRD–) complete responses (CR) in adult and pediatric and patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL) treated with KTE-C19 (anti-CD19 chimeric antigen receptor [CAR] T cells): preliminary results of the ZUMA-3 and ZUMA-4 trials (abstract). Blood. 2016;128:280z. [Google Scholar]
- 61.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015. Feb 7;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davila ML, Rivière I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014. Feb 19;6:224ra25–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brentjens RJ, Davila ML, Rivière I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013. Mar 20;5:177ra38–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pierro J, Hogan LE, Bhatla T, et al. New targeted therapies for relapsed pediatric acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2017. Aug;17:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruella M, Maus MV. Catch me if you can: leukemia Escape after CD19-directed T cell immunotherapies. Comput Struct Biotechnol J. 2016;14:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017. Jun 22;129:3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016. May 19;127:2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maude SL, Hucks GE, Callahan C, et al. Durable remissions with humanized CD19-targeted chimeric antigen receptor (CAR)-modified T cells in CAR-naive and CAR-exposed children and young adults with relapsed/refractory acute lymphoblastic leukemia. Blood. 2017;130:1319. [Google Scholar]
- 71.Yoon DH, Osborn MJ, Tolar J, et al. Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR-Ts): combination or built-in CAR-T. Int J Mol Sci. 2018. Jan 24;19:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maude SL, Hucks GE, Seif AE, et al. The effect of pembrolizumab in combination with CD19-targeted chimeric antigen receptor (CAR) T cell. J Clin Oncol. 2017;35:103. [Google Scholar]
- 73.Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45:e124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov Am Assoc Cancer Res. 2016. Jun;6:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hucks GE, Barrett D, Rheingold SR, et al. Humanized chimeric antigen receptor (CAR)-modified T cells targeting CD19 induce remissions in children and young adults with relapsed/refractory lymphoblastic leukemia/lymphoma. Cytotherapy. 2017;19:S9–S10. [Google Scholar]
- 76.Gardner R, Leger K, Annesley C, et al. Decreased rates of severe CRS seen with early intervention strategies for CD19 CAR-T cell toxicity management. Blood Adv. 2016. Dec 27;1:265–269.29296941 [Google Scholar]
- 77.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2017. Sep 19;128:LBA-6–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mackall CL, Miklos DB, Endothelial Cell CNS. Activation emerges as a driver of CART cell-associated neurotoxicity. Cancer Discov. 2017. Dec;7:1371–1373. [DOI] [PubMed] [Google Scholar]
- 79.Gust J, Hay KA, Hanafi L-A, et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with cd19 CAR-T cells. Cancer Discov. 2017. Dec 4;7:1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartmann J, Schüßler-Lenz M, Bondanza A, et al. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017. Sep;9:1183–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barrett DM, Teachey DT, Grupp SA. Toxicity management for patients receiving novel T-cell engaging therapies. Curr Opin Pediatr. 2014. Feb;26:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee DWIII, Stetler-Stevenson M, Yuan CM, et al. Long-term outcomes following CD19 CAR T cell therapy for B- ALL are superior in patients receiving a fludarabine/cyclophosphamide preparative regimen and post- CAR hematopoietic stem cell transplantation. Blood. 2016;128:218. [Google Scholar]
- 83.Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017. Oct;9:1115–1125. [DOI] [PubMed] [Google Scholar]
- 84. Porter D, Frey N, Wood PA, et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol BioMed Central. 2018. Mar 2;11:35. • Proposed grading for tisagenlecleucel-associated CRS.
- 85. Teachey DT, Hunger SP. Acute lymphoblastic leukaemia in 2017: immunotherapy for ALL takes the world by storm. Nat Rev Clin Oncol. 2018. Feb;15:69–70. • Review article describing the role of new immunotherapies in the treatment of B-ALL, including inotuzumab, blinatumomab, and tisagenlecleucel.
- 86.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014. Jul 10;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neelapu SS, Tummala S, Kebriaei P, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit “ALL.”. Nat Rev Clin Oncol. 2018. Feb 13;130:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Invest Am Soc Clin Investig. 2016. Oct 3;126:3814–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med Am Assoc Adv Sci. 2017. Jan 25;9:eaaj2013. [DOI] [PubMed] [Google Scholar]
- 90.Budde LE, Berger C, Lin Y, et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. Teague RM, editor. PLoS ONE. 2013;8:e82742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schuster SJ, Bishop MR, Tam C, et al. Global pivotal phase 2 trial of the CD19-targeted therapy CTL019 in adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) - An interim analysis. Hematol Oncol. 2017. Jun 7;35:27–7. [Google Scholar]
- 93.Li J, Li W, Huang K, et al. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol BioMed Central. 2018. Feb 13;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017. Dec 14;130:2594–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan M, Li M, Gao L, et al. Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia. J Hematol Oncol BioMed Central. 2017. Aug 29;10:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perica K, Curran KJ, Brentjens RJ, et al. Building a CAR garage: preparing for the delivery of commercial CAR T products at Memorial Sloan Kettering Cancer Center. Biol Blood Marrow Transplant. 2018. Jun;24:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.ClinicalTrials.gov NCT02906371: study of the tocilizumab optimization timing for CART19 associated cytokine release syndrome [Internet]. cited 2018 Jan. Available from: https://clinicaltrials.gov/ct2/show/NCT02906371