Abstract
The central paradigm of conventional MHC-restricted T cells is that they respond specifically to foreign peptides, while displaying tolerance to self-antigens. In contrast, it is now becoming clear that a number of innate-like T cell subsets—CD1-restricted T cells, Vγ9Vδ2 T cells, and MAIT cells—may operate by different rules: rather than focusing on the recognition of specific foreign antigens, these T cells all appear to respond to alterations to lipid-related pathways. By monitoring perturbations to the “lipidome,” these T cells may be able to spring into action to deal with physiological situations that are of self as well as microbial origin. iNKT cells are a prime example of this type of lipidome-reactive T cell. As a result of their activation by self lyso-phospholipid species that are generated downstream of blood lipid oxidation, human iNKT cells in the vasculature may respond sensitively to a variety of oxidative stresses. Some of the cytokines produced by activated iNKT cells have angiogenic effects (e.g., GM-CSF, IL-8), whereas others (e.g., IFN-γ) are pro-inflammatory factors that can propagate vascular pathology by influencing the functions of macrophages and dendritic cells. Consistent with this, evidence is accumulating that iNKT cells contribute to atherosclerosis, which is one of the most common inflammatory pathologies, and one that is integrally related to characteristics of the lipidome.
Keywords: Lipid, CD1d, iNKT, Lyso-phospholipid, Lipidome
The paradigm of lipidome-reactive T cells
CD1-restricted T cells
It has been just over two decades since the first demonstration that T lymphocytes can specifically recognize lipids as antigens: In the mid-1990s, Michael Brenner’s group demonstrated that a human αβ T cell line that was restricted by the non-classical antigen-presenting molecule CD1b specifically recognized a type of lipid (mycolic acid) produced by Mycobacterium tuberculosis (Beckman et al. 1994; Porcelli et al. 1992). In the years since, it has become clear that four of the five members of the CD1 family—CD1a, CD1b, CD1c, and CD1d—bind lipidic molecules and present them at the cell surface for recognition by human T cells (Brigl and Brenner 2004; Cohen et al. 2009; Porcelli and Modlin 1999). The remaining CD1 family member, CD1e, remains in intracellular compartments where it contributes to glycolipid processing and loading into other CD1 molecules (Angenieux et al. 2000; de la Salle et al. 2005). Crystallographic and mutational analyses have revealed that T cell recognition of lipid antigens is molecularly precise, with residues of the TCR complementarity determining loops making contacts with exposed components of the lipid or glycolipid (Luoma et al. 2014; Van Rhijn et al. 2015; Young and Moody 2006; Zajonc and Kronenberg 2009). However, while some human CD1-restricted T cells appear completely specific for microbial lipids, most are either cross-reactive with, or entirely specific for, lipid species that are of “self” origin (Van Rhijn and Moody 2015; Van Rhijn et al. 2016; Vincent et al. 2005). Thus, compared to classical MHC-restricted T cells, the CD1-restricted T cell population appears less oriented towards selective responses to foreign antigens and instead includes a large fraction that can respond to specific self-lipids.
Vγ9Vδ2 T cells
A parallel story has emerged over the last two-plus decades for a subset of human γδ T lymphocytes that bear TCRs utilizing Vγ9 and Vδ2 (Vγ9Vδ2 T cells). The TCRs of Vγ9Vδ2 T cells recognize a plasma membrane glycoprotein called butyrophilin 3A1 (BTN3A1) (Vavassori et al. 2013; Wang et al. 2013). However, Vγ9Vδ2 TCRs bind to BTN3A1 only after it has undergone structural changes wrought by its physical association with particular lipidic species (e.g., isoprenoids) (Rhodes et al. 2015; Riano et al. 2014; Sandstrom et al. 2014; Tanaka et al. 1995; Wang and Morita 2015). Isoprenoids are typically synthesized via the mevalonate pathway, which is a biosynthetic pathway present in most cell types that is responsible for producing key metabolic components such as cholesterol and other steroid lipids. Additionally, Vγ9Vδ2 T cells are activated by the presence of other lipidic compounds that are synthesized only by bacteria and protozoa that posses a non-mevalonate (alternative) biosynthesis pathway (Belmant et al. 1999; Puan et al. 2007). The compounds produced by microbes via the non-mevalonate pathway are highly potent activators of Vγ9Vδ2 T cells, whereas the isoprenoid species produced by mammalian cells is less potent at inducing Vγ9Vδ2 T cell activation (Morita et al. 2007; Wang et al. 2011). Vγ9Vδ2 T cells thus are extremely sensitive to the presence of microbial infections that result in the accumulation of potently activating “non-self” isoprenoids, but they can also be activated by cellular events (e.g., rapid proliferation, neoplastic transformation) that are associated with the upregulation of mammalian isoprenoid biosynthesis. Notably, since statins interrupt the mevalonate biosynthesis pathway upstream of the step leading to the production of the key isoprenoid compound, these drugs likely inhibit the activation of Vγ9Vδ2 T cells in response to endogenous cellular processes, while not affecting their responses to microbial infections.
MAIT cells
Very recently, a T cell population called mucosal-associated-invariant T (MAIT) cells has also been shown to respond to lipid-related cues. MAIT cells are restricted by the non-classical antigen-presenting molecule MR1 (Treiner et al. 2003). MR1 molecules bind flavonoids (e.g., B-group vitamins) and present these at the cell surface (Kjer-Nielsen et al. 2012; Reantragoon et al. 2012). The TCRs of MAIT cells bind with high affinity to MR1 presenting certain B-vitamin derivatives (e.g., riboflavin metabolites) leading to activation of the MAIT cell, whereas related MR1-binding compounds (e.g., folate metabolites) provide sub-optimal TCR stimulation that inhibits the MAIT cells (Patel et al. 2013). Thus, MAIT cells may mediate early responses to bacterial pathogens (e.g., Salmonella and E. coli species) that specifically synthesize the activating riboflavin pathway compounds, while becoming tolerized by bacteria that produce only folate pathway compounds (Birkinshaw et al. 2014). However, since non-pathogenic bacteria (e.g., Bacillus subtilis which is often present in our gut microbiota) can also synthesize riboflavin, and flavonoids produced by plants can be obtained through diet and are then disseminated throughout the body, MAIT cells may also become activated in non-infection related contexts. Notably, the two types of B-group vitamins distinguished by MAIT cells are also critical for distinct aspects of lipid metabolism in host cells: riboflavin plays a role in β-oxidation of fatty acids (i.e., breakdown of fats to generate energy), whereas folate plays a unique role as a catalytic substrate in the chemical transfer of one-carbon units needed for certain nucleotide and amino acid biosynthetic pathways (da Silva et al. 2014). Hence, the presence of riboflavin vs. folate pathway metabolites may be linked to distinct metabolic activities of host cells as well as signaling the presence of specific bacterial species.
Responding to perturbations to the lipidome
The emerging picture from these three types of T cells—CD1-restricted T cells, Vγ9Vδ2 T cells, and MAIT cells—thus suggests that they comprise a novel T lymphocyte compartment that can be activated by a variety of molecular alterations to the lipidome, rather than being highly specific for foreign compounds that are unique to microbial pathogens. According to this perspective, the T cell populations belonging to this compartment each become activated by characteristic metabolic changes, whether these changes result from microbial infection, changes to the composition of non-pathogenic microbial colonists (microbiota), or host-intrinsic processes such as neoplastic transformation or normal developmental events. By responding to lipidome-alteration cues, members of this T cell compartment may thus be able to contribute not only to host defense against infection but also to inflammatory processes affecting tolerance and autoimmunity, as well as to the regulation of host developmental processes and the control of neoplastic transformation.
The unifying principle of this lipidome-monitoring T cell compartment might therefore be that it serves as an immune branch that is oriented towards controlling metabolic imbalances, regardless of whether these result from “exogenou” or “endogenou” processes. As a logical extension of this, T cells from this compartment might be expected to play particularly important roles in metabolic diseases. While there is as yet little information on the roles of MAIT cells, Vγ9Vδ2 T cells, and most CD1-restricted T cells in metabolic diseases, one subset, CD1d-restricted invariant Natural Killer T (iNKT) cells, has been comparatively well studied. In the following sections, we will focus on endogenous pathways that drive human iNKT cell activation in the blood, and which may ultimately determine their contribution to atherogenesis, which is one of the most common metabolism-associated pathologies.
iNKT cells: a flagship lipidome-reactive T cell subset
NKT cells were originally identified as a murine lymphocyte population that expressed CD3, a clear indicator of their status as T cells, but that differed from conventional T cells in that they co-expressed CD161 (also known as NK1.1), a marker that is characteristic of NK cells (Bix and Locksley 1995). The molecular definition of NKT cells was refined by the finding that most utilize a canonical TCRα chain rearrangement (Vα14-Jα18 in mice, Vα24-Jα18 in humans) that is paired with a limited set of TCRβ chains (Koseki et al. 1990; 1991; Porcelli et al. 1996). Further analysis revealed that T cells using this canonical TCRα chain are restricted by CD1d antigen-presenting molecules (Bendelac et al. 1995; Exley et al. 1997; Lantz and Bendelac 1994). Since other CD1d-restricted T cells were identified that possessed diversely recombined TCRα and β chains (Behar et al. 1999; Cardell et al. 1995; Exley et al. 2001), the canonical TCRα subset was given the moniker of “invariant” NKT (iNKT) cells.
iNKT cells can mediate either tolerogenic or pro-inflammatory effects
Functionally, iNKT cells might best be characterized as a “jack-of-all-trade” lymphocyte subpopulation. They initially attracted attention because they were responsible for the early burst of IL-4 cytokine secretion observed in mice after injection of anti-CD3 antibodies (Bendelac et al. 1994; Yoshimoto et al. 1995), but it was soon appreciated that they can rapidly produce large amounts of both TH1 and TH2 cytokines (Behar et al. 1999; Brown et al. 1996; Chen and Paul 1997; Smiley et al. 1997), and more recently, it has been demonstrated that some iNKT cells can produce TH17-pathway cytokines including IL-17 and IL-22 (Coquet et al. 2008; Goto et al. 2009). From analyses of their gene expression and thymic development, it has become clear that murine iNKT cells are divided into sub-lineages showing TH1-, TH2-, or TH17-biased cytokine profiles and expressing the transcription factors T-bet, GATA-3, and RORγT, correspondingly (Lee et al. 2013). Moreover, in mice, these distinct iNKT sub-lineages segregate to different peripheral tissue locations (Lee et al. 2015). Whether human iNKT cells are similarly comprised of distinctly polarized sub-lineages remains unclear. However, it is clear that human peripheral blood iNKT cells can produce both TH1 and TH2 cytokines directly ex vivo (Gumperz et al. 2002). Exposure to IL-12p70 drives human iNKT cells towards a highly TH1-restricted phenotype (Brigl et al. 2003), whereas exposure to TGF-β, IL-1β, and IL-23 induces TH17 cytokine production (Moreira-Teixeira et al. 2011). Thus, both human and murine iNKT cells are multifaceted cytokine producers, with the specific cytokine pathway that predominates in a given context likely depending on the local environment. Consistent with this potential for pleiomorphic functionality, iNKT cells act to inhibit excessive inflammation in autoimmune disease contexts (Ronchi and Falcone 2008; Wu and Van Kaer 2009), whereas in many microbial infections, they enhance inflammatory responses and thus contribute to improved host defense (Brigl and Brenner 2010; Tupin et al. 2007).
Antigenic specificity of iNKT cells
A key breakthrough in our molecular understanding of iNKT cells came with the demonstration that they recognize CD1d-mediated presentation of specific lipids as antigens. The first indication of the specificity of iNKT cells for lipidic antigens came from the identification of an unusual class of glycolipids called α-glycosylsphingolipids (α-GSLs) as potent antigens for both murine and human iNKT cells (Kawano et al. 1997; Spada et al. 1998). The prototypical antigen of this type, called α-galactosylceramide (α-GalCer), was originally isolated from tissue samples of the marine sponge Agelas mauritianus (Kawano et al. 1997). Subsequently, bacterial glycolipids were identified that contained similar α-linked sugar moieties, and were shown to specifically activate iNKT cells, although less potently than α-GalCer (Kinjo et al. 2005; 2006; Mattner et al. 2005).
However, starting with the first experiments showing iNKT cell restriction by CD1d (Bendelac et al. 1995), it was apparent that iNKT cells also display functional responses (e.g., cytokine secretion) to CD1d+ APCs in the absence of exogenously added antigens. Based on experiments demonstrating a key role of the CD1d cytoplasmic tail (which determines intracellular trafficking of CD1d molecules and thus access to cellular compartments containing distinct types of endogenous lipid antigens), the observed iNKT cell autoreactivity was postulated to be due to CD1d-mediated presentation of specific self-lipids (Brossay et al. 1998; Chiu et al. 1999). Directly supporting this hypothesis was the demonstration that a murine iNKT cell hybridoma was unable to recognize recombinant CD1d molecules unless a cellular lipid extract was added (Gumperz et al. 2000). From these experiments and many others, it has now become firmly established that iNKT recognition of specific self-lipids is sufficient to cause their functional activation and is likely a key aspect of their distinctive immunological properties (Bendelac et al. 2001; Kronenberg and Rudensky 2005).
The search for self-antigens that activate iNKT cells
A central question in the field has been to identify the self-lipids recognized by iNKT cells. The potent ability of α-GSLs to activate all iNKT cells raised the possibility that lipids of this type might not simply represent a foreign molecular pattern but might also be responsible for iNKT cell self-antigenic activation (Gumperz and Brenner 2001). However, since mammalian glycosylceramide synthases are invertases and thus are not capable of producing α-anomeric linkages, and it was found that β-glucosylceramide synthase deficient cells were unable to stimulate iNKT cells (Stanic et al. 2003), attention focused on the role of β-linked glycolipids. Mice that lack a lysosomal glycosidase (hexosaminidase b, or Hex b) were shown to be deficient in iNKT cells (Zhou et al. 2004), and this was related to iNKT cell recognition of a β-linked glycolipid lipid called isoglobotrihexosylceramide (iGb3) (Zhou et al. 2004). However, despite the ability of iGb3 to activate iNKT cells and to be specifically recognized as a CD1d-presented ligand by murine iNKT TCRs (Zajonc et al. 2008), deletion of the α-galactosyl transferase enzyme (iGb3S) that is specifically responsible for the synthesis of the iGb3 glycolipid did not appear to affect murine iNKT cell numbers or peripheral function (Porubsky et al. 2007). Moreover, humans appear to lack the ability to generate the iGb3 glycolipid due to the absence of a functional iGb3 synthase gene (Christiansen et al. 2008), and iNKT cells are not able to recognize iGb3 when it is presented by human CD1d molecules (Sanderson et al. 2013). As a result of contraindicative findings of this type, the search for endogenous glycolipids that activate human and murine iNKT cells has continued and has recently led to the identification of trace amounts of α-GSLs in thymocytes and dendritic cells that potently activate iNKT cells (Kain et al. 2014; 2015).
Self antigen strength vs. abundance
The highly potent self α-GSLs recognized by iNKT cells are thought to be constitutively produced, but tightly regulated by catabolic processes, such that they are generally only present at very low levels (Kain et al. 2014; 2015). In addition to this type of antigen, a fraction of human iNKT cells are able to recognize certain self-lipids that are highly abundant, but that provide a comparatively weak antigenic stimulus (Chamoto et al. 2016; Fox et al. 2009). Self-lipids that are weak agonists may play important roles in the responses of iNKT cells by contributing to the maintenance of their poised-effector status, which is characterized by the ability to become activated in a TCR-independent manner through exposure to cytokines such as IL-12 and IL-18 (Wang et al. 2012). Moreover, recognition of self-antigens that are upregulated as a result of cellular metabolic changes may also allow iNKT cells to participate in “sterile” inflammatory responses (i.e., inflammatory responses that are not dependent on the presence of microbial compounds) (Zeng et al. 2013).
iNKT self-antigens as indicators of metabolic processes
While the range of self-lipids recognized by iNKT cells is not yet fully characterized, they have been found to respond specifically to two structurally related types of self-lipid that are integrally linked to metabolic processes. The first of these, lyso-phosphatidylcholine (LPC), is produced during normal lipid metabolism-related cellular activities and is also markedly upregulated during inflammatory responses, since it is generated as a byproduct from cellular biosynthesis of eicosanoid lipid mediators (Funk 2001). LPC species were identified within the pool of lipids eluted from human CD1d molecules (Cox et al. 2009; Yuan et al. 2009), indicating that these single acyl chain species can bind to human CD1d molecules despite the presence of di-acylated species, which made up much of the ligand pool. LPC species found in the CD1d-ligand pool elicited clear CD1d-dependent responses from human peripheral blood iNKT cells, whereas there was little or no response to other lipids from the CD1d-ligand pool (Fox et al. 2009). The iNKT cell response to LPC appeared specific to the chemical head group of this lipid (choline), since lyso-phospholipids containing different head groups (e.g., lyso-phosphatidylethanolamine and lyso-phosphatidic acid) were not recognized, whereas another lyso-lipid with a choline head group (lyso-sphingomyelin) did stimulate CD1d-dependent iNKT cell responses (Fox et al. 2009). Furthermore, a crystal structure was solved of an iNKT TCR in complex with CD1d-bound LPC that showed residues of the TCR making specific contacts with the LPC head group (Lopez-Sagaseta et al. 2012). Thus, a fraction of the human peripheral blood iNKT cell population appears to specifically recognize LPC as a CD1d-presented antigen.
In contrast, murine iNKT cells have not been found to specifically recognize LPC, although certain murine CD1d-restricted T cells with non-canonical TCR rearrangements (“type II NKT cell”) are able to recognize this compound (Maricic et al. 2014). Additionally, iNKT cell frequency was significantly reduced in mice lacking lysosomal phospholipase A2 (enzymes that cleave di-acylated lipid species to produce lyso-phospholipids), and APCs deficient in lysosomal phospholipase A2 showed reduced ability to activate some murine iNKT cells via CD1d presenting endogenous antigens (Paduraru et al. 2013). Thus, while LPC may not serve as a specific antigen for murine iNKT cells, a key enzyme involved in the generation of lyso-phospholipids does appear to play a role in their development and functional responses to self-antigens, and there are also non-invariant CD1d-restricted T cells in mice that recognize this antigen.
Further supporting an important role for lyso-phospholipids in iNKT cell responses is the finding that murine iNKT cells can recognize lyso-plasmalogens. Plasmalogens are glycero-phospholipids that are synthesized within peroxisomes (cellular organelles involved in catabolism of very long-chain fatty acids) and that have an unusual structure in which one or two alkyl chains are attached to the glycerol molecule via ether-containing linkages. Experiments using plasmalogen-deficient cells have indicated they play important roles in intracellular cholesterol transport following exposure to LDL or HDL particles (Mandel et al. 1998; Munn et al. 2003). Analysis of lipids extracted from murine thymocytes revealed CD1d-dependent recognition of two lyso-plasmalogen species, plasmalogen lyso-phosphatidylethanolamine (pLPE) and ether lyso-phosphatidic acid (eLPA) by murine iNKT cells (Facciotti et al. 2012). These lipids share structural similarity with LPC in that they are also glycero-phospholipids that only contain a single hydrocarbon chain, but they differ from LPC in the chemical nature of the head group (ethanolamine or phosphatidic acid vs. choline) and in having an ether bond between the acyl chain and the glycerol. Pointing to the importance of these compounds in vivo, mice lacking an enzyme required for the biosynthesis of plasmalogens showed altered iNKT cell maturation and diminished iNKT cell frequency in both the periphery and thymus (Facciotti et al. 2012).
Plasmalogen species were also identified in the human CD1d ligand pool (Cox et al. 2009), consistent with a potential role as human iNKT antigens. A recent analysis of a diverse repertoire of human iNKT cell TCRs revealed that a fraction bound to CD1d molecules presenting either LPC or pLPE, although none bound to CD1d presenting eLPA (Chamoto et al. 2016). Thus, in addition to recognition of lyso-phospholipids with a choline head group (e.g., LPC), human iNKT cells may recognize lyso-plasmalogens that contain an ethanolamine head group (i.e., pLPE). It is not yet clear whether lyso-plasmalogens that have a choline head group, such as platelet-activating factor (discussed below), are antigenic for human iNKT cells. However, given their ability to recognize choline-containing lyso-phospholipids and at least one type of lyso-plasmalogen, it seems likely that human iNKT cells may also recognize plasmalogen forms of LPC.
Generation of iNKT cell self-antigens in the blood
LPC production as a reflection of oxidative stress levels
LPC is typically present at high concentrations (>100 μM) in the blood and is mainly found in high density lipoprotein (HDL) particles or bound to serum albumin molecules (Croset et al. 2000; Serna et al. 2015). LPCs are produced by cleavage of the sn-1 or sn-2 acyl chain bonds of phosphatidylcholine (PC), which can occur either through chemical oxidation or through the hydrolytic activity of phospholipase A1 or A2 enzymes (PLA1 or PLA2), respectively. It is perhaps not surprising that LPC is a major component of HDL, since these particles also contain most of the PC in the plasma (Serna et al. 2015), and thus they provide an abundance of substrate for the generation of LPC by PLA enzymes. One of the major PLA enzymes that acts on HDL particles is called endothelial lipase (EL) and is expressed by activated vascular endothelial cells (Fig. 1). This enzyme has been found to function as a phospholipase A1 (PLA1) that cleaves PC embedded in HDL particles, releasing LPC (Choi et al. 2002; Riederer et al. 2012). Since activation of vascular endothelial cells results mainly from exposure to oxidized lipids or inflammatory cytokines, this pathway of LPC production may directly reflect the occurrence of oxidative stress in the blood.
Fig. 1.
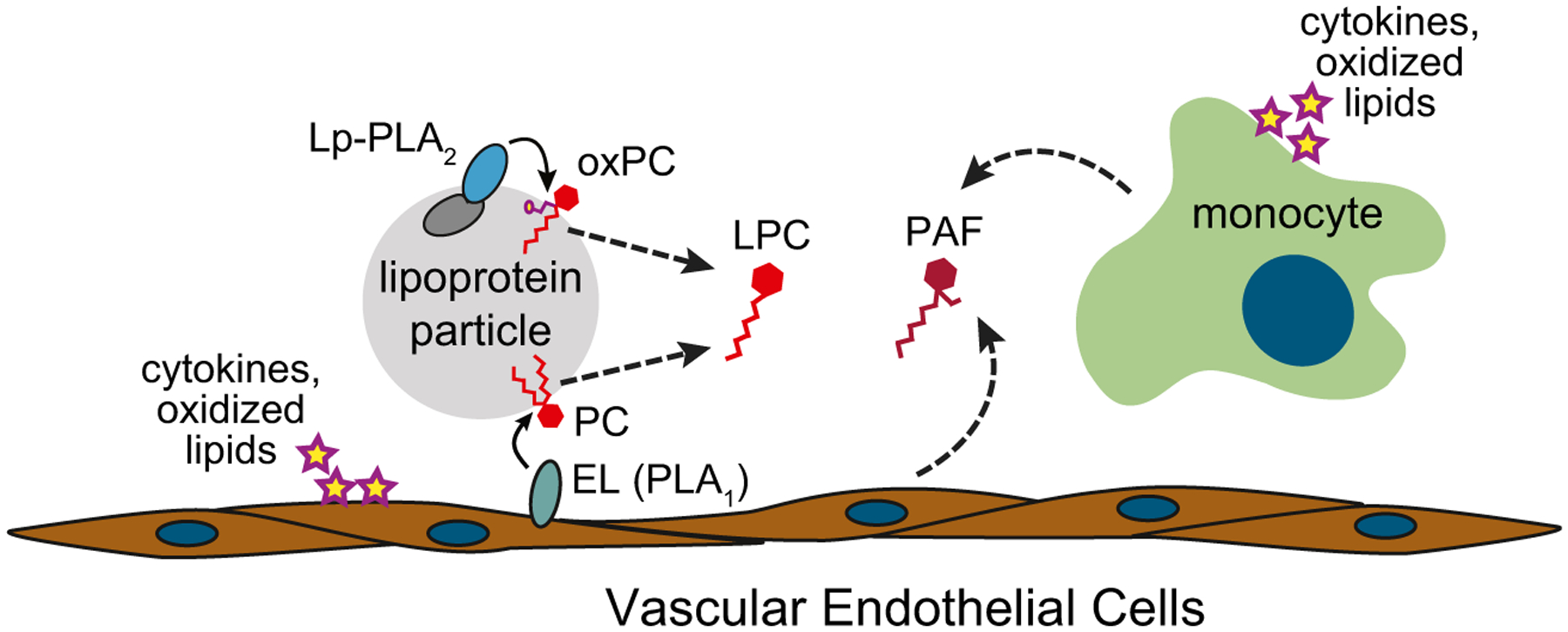
Generation in the blood of lyso-phosphatidylcholine (LPC) and platelet-activating factor (PAF), which are potential self-antigens for human iNKT cells. Exposure to inflammatory cytokines or oxidized lipids causes endothelial cell surface expression of endothelial lipase (EL), which is a phospholipase A1 (PLA1) enzyme that cleaves phosphatidylcholine (PC) molecules embedded in HDL or LDL lipoprotein particles. Also, typically associated with HDL and LDL particles is lipoprotein-associated phospholipase A2 (Lp-PLA2), which cleaves oxidized PC molecules to yield LPC. Additionally, monocytes and endothelial cells release platelet-activating factor (PAF), a lyso-phospholipid with structural similarities to LPC
LPC is also generated in the blood by a specific PLA2 enzyme (lipoprotein-associated PLA2 or Lp-PLA2) that associates with LDL and HDL via physical interactions with apolipoproteins embedded in the surface of these lipoprotein particles (Cao et al. 2013; Stafforini et al. 1999; Steinbrecher and Pritchard 1989). The Lp-PLA2 enzyme preferentially hydrolyzes oxidized PC molecules (oxPC) and PC species that contain short acyl chains and acts on oxidized LDL to release LPC (Silva et al. 2011). Hence, this pathway produces LPC as a byproduct of processes that clear oxidized PC molecules from the plasma. While a certain amount of oxPC clearance probably occurs continuously giving rise to a steady state supply of LPC, the levels of LPC produced through this pathway may also directly reflect the amount of oxidative stress, as more LPC will likely be generated under conditions where more oxPC is produced and then cleared by Lp-PLA2.
Elevated plasma LPC in chronic inflammation
Consistent with the potential for LPC to be released via multiple inflammation-associated pathways, LPC levels in the blood and other bodily fluids are often greatly elevated under conditions of chronic inflammation, including certain cancers (Sevastou et al. 2013). A biochemical analysis of ligands bound by recombinant CD1d molecules that were incubated in blood plasma obtained from human multiple myeloma patients revealed several species of LPC as major components of the CD1d ligand pool (Chang et al. 2008). These results suggest that under conditions where blood LPC levels are elevated, this antigen is able to load directly into CD1d molecules without requiring the aid of intracellular lipid loading machinery. Thus, although the binding of LPC to CD1d glycoproteins is predicted not to be highly stable (since it only has a single acyl chain to anchor it within the CD1d hydrophobic binding pocket) and it may therefore have a comparatively high off rate, LPC may also load rapidly into cell surface CD1d molecules of antigen-presenting cells. As a result of this instability, LPC presentation by CD1d+ APCs in the blood may sensitively reflect its current plasma concentration.
Platelet-activating factor
As noted above, in addition to LPC, lyso-plasmalogens with a choline head group may also be antigenic for human iNKT cells. The major plasmalogen species found in the blood is platelet-activating factor (PAF, also known as acetyl-glyceryl-ether-phosphorylcholine). PAF is a highly biologically active inflammatory lipid mediator that is produced by vascular endothelial cells and by many immune cells, including monocytes (Fig. 1). Two distinct pathways give rise to PAF: de novo biosynthesis and remodeling of existing di-acylated phospholipids (usually PC) by PLA2 and acetyl transferase enzymes (Snyder 1988). While PAF is continuously produced at low levels (mainly through the biosynthesis pathway), the remodeling pathway becomes rapidly activated in response to a variety of inflammatory agents, leading to increased PAF generation (Snyder et al. 1996). Blood levels of PAF are typically limited by the action of acetylhydrolase enzymes, including Lp-PLA2 (the enzyme noted above that associates with circulating LDL particles). Removal of the acetyl group at the sn-2 position of PAF by Lp-PLA2 yields a plasmalogen form of LPC that lacks many of the pro-inflammatory signaling effects associated with the starting PAF molecule (Marathe et al. 2014), and thus under normal circumstances, Lp-PLA2 activity may keep the inflammatory effects of PAF from spiraling out of control. While not yet clear, it is possible that the product resulting from the hydrolysis of PAF by Lp-PLA2 (plasmalogen-LPC) remains antigenic for iNKT cells.
Delivery of lipid antigens by blood transport systems
The vascular system is responsible for the transport of lipids (e.g., cholesterol, triglycerides, and fatty acids) throughout the body, and thus the blood contains multiple types of lipid-binding proteins and lipoprotein particles. A synthetic glycolipid (α-GalCer) that is a potent antigen for iNKT cells was found to be incorporated into very low density lipoprotein (VLDL) particles from human serum as a result of binding specifically to apolipoprotein E (ApoE) (van den Elzen et al. 2005). The ApoE-bound lipid antigen was taken up by human monocyte-derived DCs via an ApoE receptor-dependent process that led to highly efficient antigen presentation by CD1d molecules (van den Elzen et al. 2005). In contrast, an analysis of α-GalCer uptake by APCs in a murine model found that a lipid-binding protein in the serum called fatty acid amide hydrolase (FAAH) was responsible for enhancing presentation by CD1d molecules (Freigang et al. 2010). In the case of FAAH, it appeared that soluble FAAH may transfer bound α-GalCer directly to cell surface CD1d molecules, rather than utilizing receptor-mediated endocytosis (Freigang et al. 2010). These studies have clearly delineated the ability of lipid transport factors in the blood to enhance the uptake and CD1d-mediated presentation of a synthetic glycolipid to iNKT cells (Fig. 2). While not yet experimentally determined, it seems highly likely that CD1d-mediated presentation of other types of lipids (e.g., LPC) may also be enhanced by blood-intrinsic lipid transporters.
Fig. 2.
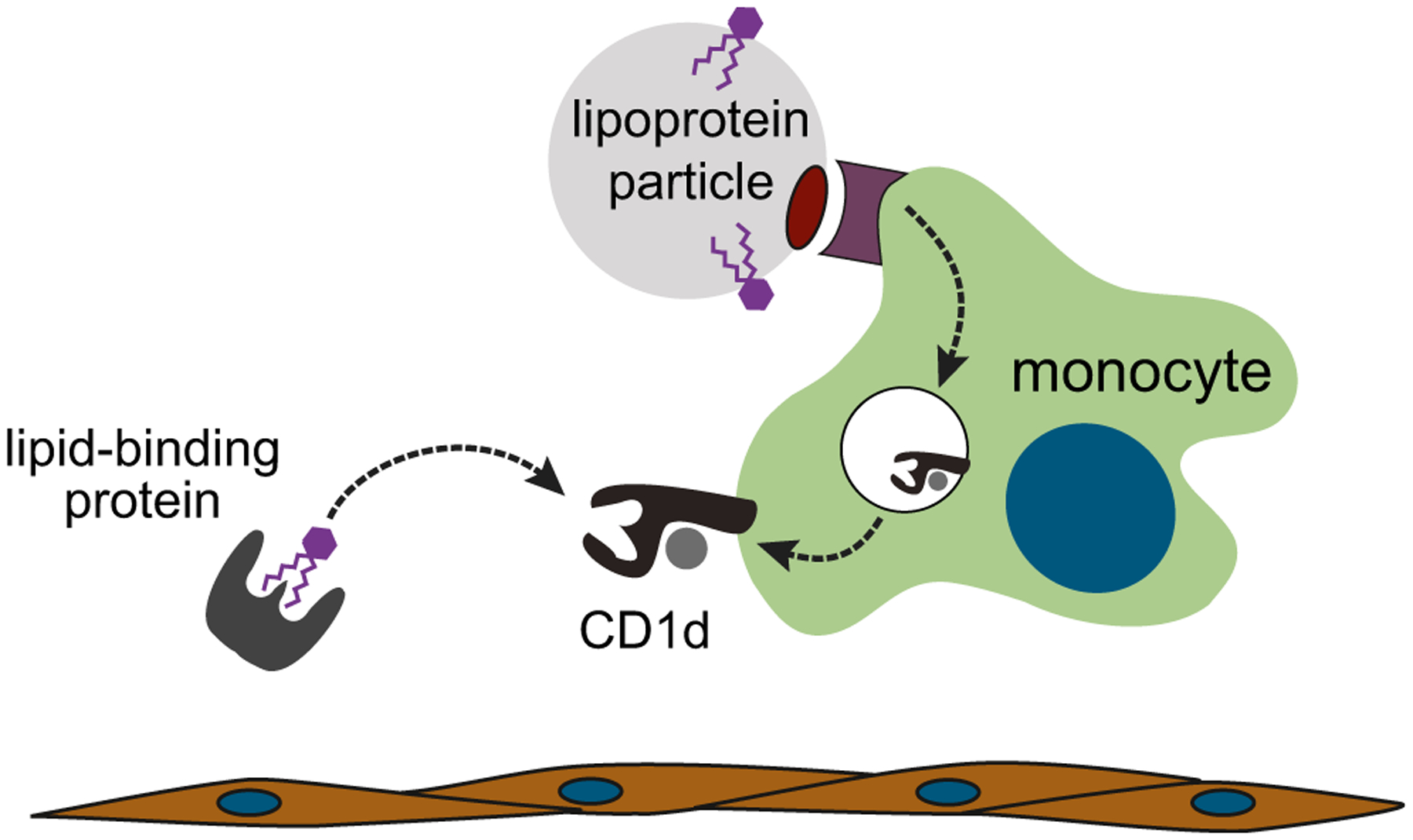
The blood contains multiple lipid transporters that can enhance the uptake and presentation of lipid antigens by CD1d on antigen-presenting cells. α-GalCer, an antigen recognized by iNKT cells that contains two lipid tails, can be taken up by very low density lipoprotein (VLDL) particles and internalized by antigen-presenting cells via the apolipoprotein E (ApoE) receptor, where it is efficiently loaded into CD1d molecules for presentation at the cell surface. Alternatively, α-GalCer can bind to lipid-binding proteins such as fatty acid amide hydrolase that transfer the lipid directly to cell surface CD1d molecules
Inflammatory activation of vascular iNKT cells
Intravital microscopy analyses of murine tissues have revealed that iNKT cells randomly crawl along vascular endothelial surfaces in the steady state (Geissmann et al. 2005; Lee et al. 2010; Wong and Kubes 2013). In the event that iNKT cells detach from the vascular endothelium, they are seen to rapidly reattach a short way downstream, suggesting that they are essentially resident on the vascular endothelial surfaces and not simply occasionally attaching while being transported through the blood stream (Paul Kubes personal communication). From parabiosis experiments, in which the vascular systems of two different mice are surgically connected, it became clear that the iNKT cells in the vascular beds of the liver are indeed stably resident at this site (and not simply preferentially lingering there while circulating through the blood), since liver iNKT cells from one joined mouse do not show up in the liver tissue of the other mouse (Thomas et al. 2011). The residency of iNKT cells within the vascular sinusoids of the liver appeared to be due to their unusually high levels of the adhesion molecule LFA-1, a feature that is conferred by iNKT expression of the PLZF transcription factor (Thomas et al. 2011).
Based on their elevated LFA-1 expression, it seems likely that iNKT cells may be selectively retained at any vascular sites where endothelial cell expression level of the LFA-1 ligand ICAM-1 is elevated. Vascular endothelial cells rapidly upregulate ICAM-1 in response to a variety of stimuli, includeing inflammatory cytokines (e.g., IL-1, TNF) and oxidized lipids (Fig. 3). The presence of elevated levels of adhesion molecules on vascular endothelial cells is a key signal that causes monocytes to stop rolling along the endothelial surface and to attach more securely (Gerhardt and Ley 2015). Given that monocytes are the most abundant CD1d+ cell type within the blood, it thus seems reasonable to suppose that activated vascular endothelial cells that have upregulated ICAM-1 might provide a focal point at which iNKT cells might meet and interact with monocytic antigen-presenting cells (Fig. 3). Our studies of the interactions of human iNKT cells with freshly isolated blood monocytes have demonstrated that in the absence of added stimulation (e.g., from microbial compounds or pro-inflammatory cytokines), the main cytokines secreted by iNKT cells are GM-CSF and IL-13, and there is very little production of IFN-γ (Hegde et al. 2007; Wang et al. 2008). Since GM-CSF has been previously shown to promote endothelial cell proliferation and migration and to enhance the repair of wounding to endothelial cell layers (Bussolino et al. 1991), the cytokine output from vascular interactions between iNKT cells and monocytes in the absence of an inflammatory cytokine milieu might help to maintain endothelial regeneration and repair. In contrast, in the presence of strong antigenic signals or of pro-inflammatory cytokines such as IL-12p70, human iNKT cells dramatically upregulate their secretion of IFN-γ (Brigl et al. 2003; Wang et al. 2008). Thus, in the context of “danger” signals such as increased antigenic stimulation or inflammatory cytokines, iNKT cells assume a highly pro-inflammatory cytokine secretion pattern.
Fig. 3.
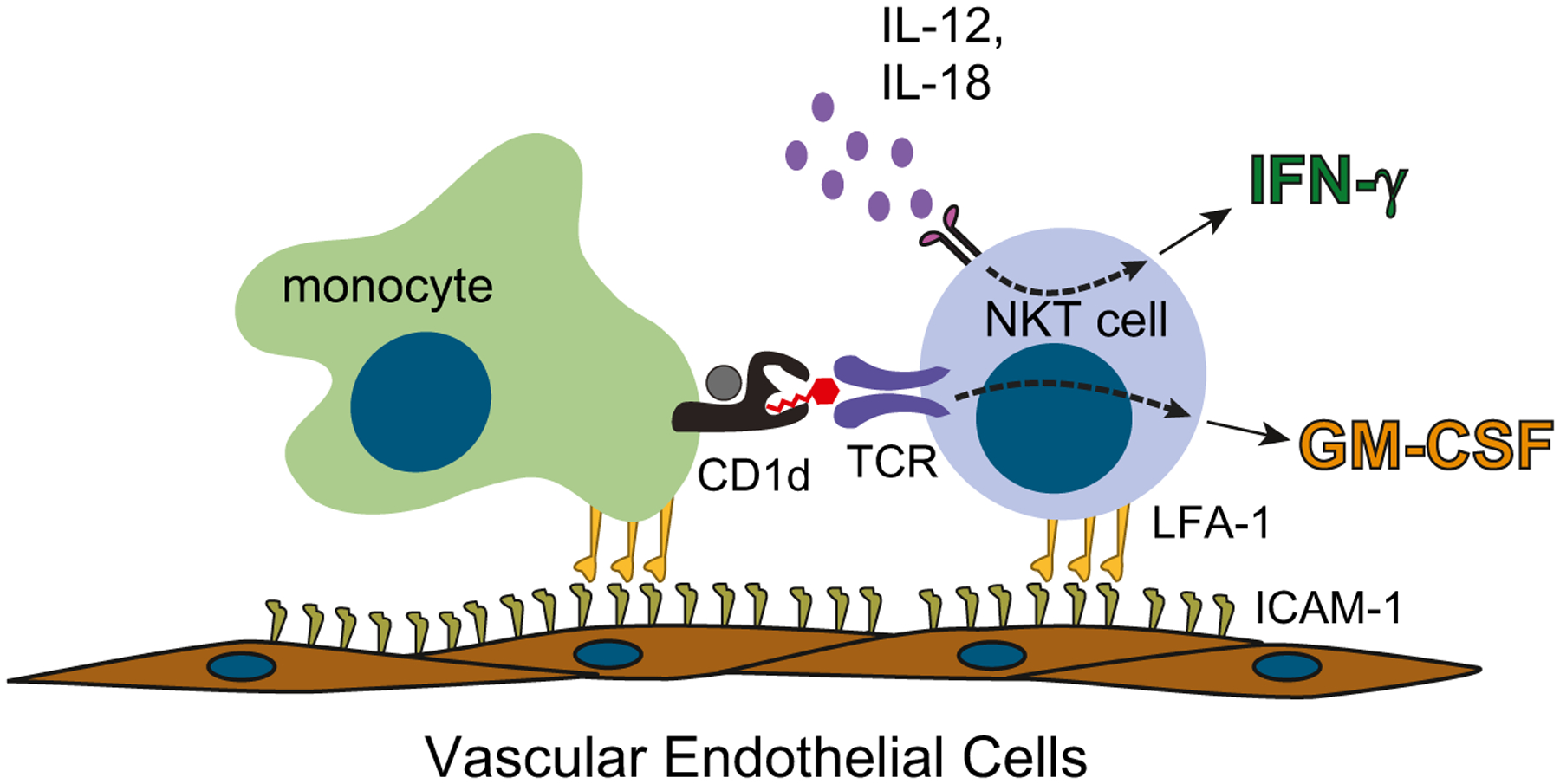
Recruitment of iNKT cells and blood monocytes to endothelial surfaces that have upregulated intercellular adhesion molecules 1 (ICAM-1). iNKT cells express high levels of lymphocyte function associated antigen 1 (LFA-1), which is the ICAM-1 adhesion ligand, and therefore are likely to be efficiently recruited to endothelial sites where ICAM-1 is upregulated. Monocytes are the most abundant CD1d + antigen-presenting cells in human blood and are also efficiently recruited to activated endothelial surfaces. In the absence of pro-inflammatory cytokines, iNKT cell activation by self-antigens leads them to mainly secrete GM-CSF. However, in the presence of inflammatory cytokines such as IL-12 and IL-18, iNKT cells efficiently produce IFN-γ
This change in iNKT cell function towards a pro-inflammatory phenotype may have particularly critical effects when it occurs on vascular endothelial surfaces. A telling example is the role of iNKT cells in ischemia reperfusion pathologies. In particular, iNKT cells have been found to play a key part in vascular injury in models of sickle cell disease (SCD), where endothelial damage occurs as a result of the occlusion of small vessels by rigid and misshapen red blood cells that form following hypoxic stress. In murine SCD models, iNKT cells have been found to amplify vascular pathology by production of pro-inflammatory mediators such as IFN-γ and the chemokine CXCR3 (Wallace et al. 2009). Moreover, human SCD patients have been found to possess elevated frequencies of activated iNKT cells in the circulation (Field et al. 2011), and a subset of human iNKT cells (CD4+ iNKTs) undergo rapid activation of NFkB during vaso-occlusive crises (Lin et al. 2013). These findings highlight the ability of iNKT cells to contribute to acute inflammation within the vasculature. However, they may also contribute to chronic forms of vascular inflammation, including the processes leading to atherosclerosis.
iNKT cells and atherosclerosis
Atherosclerosis is a condition involving the generation of fatty plaques in arterial cell walls (intima) that are infiltrated by a variety of immune cells, including myelomonocytic cell types (e.g., monocytes, macrophages, DCs) and lymphocytes. While the precise factors and sequence of events leading to the establishment of arterial plaques remains a subject of active investigation, it is known that endothelial cell contact with oxidized lipids leads to their increased cell surface expression of adhesion molecules (Weber et al. 1999), which in turn leads to the capture of leukocytes. Adhered monocytes migrate from the luminal endothelial surface into the arterial wall, differentiating into tissue macrophages that engulf lipidic particles deposited at the site. Invasion of the arterial wall by immune cells produces a local inflammatory environment and results in the formation of a plaque that is typically characterized by the presence of a necrotic core with evidence of calcification (Otsuka et al. 2014).
T lymphocytes are thought to play an important role in the development of atherosclerosis, as the T cell cytokine IFN-γ is known to be a critical signal promoting the atherogenic activeities of macrophages and DCs (Voloshyna et al. 2014). In recent years, it has become clear that iNKT cells may make particularly important contributions to atherogenesis. Findings from murine model systems pointing to a key role for iNKT cells in the etiology of atherosclerotic plaques include the following: (i) iNKT cells are found infiltrating the atherosclerotic lesions in ApoE−/− or LDLr−/− mice (Aslanian et al. 2005; Nakai et al. 2004), and laser capture microdissection of atherosclerotic lesions followed by RT-PCR showed that expression of IFN-γ was significantly reduced in lesions from mice lacking iNKT cells (Rogers et al. 2008). (ii) ApoE−/− or LDLr−/− mice that are deficient in iNKT cells show reduced atherosclerotic lesion size compared to those that possess normal iNKT cell frequencies (Aslanian et al. 2005; Major et al. 2004; Nakai et al. 2004; Rogers et al. 2008; Tupin et al. 2004). (iii) Increasing the iNKT cell frequency by adoptive transfer or the use of transgenic mice results in increased atherosclerotic lesion size (Li et al. 2015b; Subramanian et al. 2013; To et al. 2009; VanderLaan et al. 2007). (iv) Specific activation of iNKT cells in vivo by administration of α-GalCer also results in increased atherosclerotic lesion size (Major et al. 2004; Nakai et al. 2004; Tupin et al. 2004). (iv) Specifically, inhibiting iNKT cell activation by administering a synthetic compound that blocks their recognition of CD1d results in ameliorated atherosclerosis and reduced plaque pathology (Li et al. 2015a). Consistent with these findings from murine models, analyses of human atherosclerotic plaques have also revealed a substantial frequency of iNKT cells (comprising up to 3 % of the infiltrating T cells), as well as of CD1d+ APCs (Bobryshev and Lord 2005; Chan et al. 2005; Kyriakakis et al. 2010; Melian et al. 1999). The results from experimental models and analyses of primary human tissues thus provide strong evidence that iNKT cells can play an important role in the pathogenesis of arteriosclerotic vascular disease.
Concluding thoughts
The emerging concept of a T cell compartment comprised of subsets that are activated by alterations in lipid metabolism, whether these originate from host or microbe, may provide a particularly valuable lens for our understanding of human metabolic disease. As a case in point, it is becoming increaseingly clear that iNKT cells contribute to cardiovascular pathology, yet fundamental questions remain about the physiological events involved in their vascular activation. These include whether they are mainly activated by rare self-lipids that are strong agonists (e.g., endogenous α-GSLs) or by compounds that are comparatively weak agonists but that may be particularly abundant in vascular settings (e.g., LPC), the role of oxidative stress in generating lipid antigens recognized by iNKT cells, and whether iNKTs have beneficial homeostatic effects in the vasculature, such as promoting endothelial regeneration or repair. Understanding these issues will be critical in moving forward with therapeutic approaches targeting iNKT cells.
References
- Angenieux C, Salamero J, Fricker D, Cazenave JP, Goud B, Hanau D, de La Salle H (2000) Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J Biol Chem 275:37757–37764 [DOI] [PubMed] [Google Scholar]
- Aslanian AM, Chapman HA, Charo IF (2005) Transient role for CD1d-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler Thromb Vasc Biol 25:628–632 [DOI] [PubMed] [Google Scholar]
- Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB (1994) Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 372:691–694 [DOI] [PubMed] [Google Scholar]
- Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB (1999) Diverse TCRs recognize murine CD1. J Immunol 162:161–167 [PubMed] [Google Scholar]
- Belmant C, Espinosa E, Poupot R, Peyrat MA, Guiraud M, Poquet Y, Bonneville M, Fournie JJ (1999) 3-Formyl-1-butyl pyrophosphate A novel mycobacterial metabolite-activating human gammadelta T cells. J Biol Chem 274:32079–32084 [DOI] [PubMed] [Google Scholar]
- Bendelac A, Killeen N, Littman DR, Schwartz RH (1994) A subset of CD4+ thymocytes selected by MHC class I molecules. Science 263:1774–1778 [DOI] [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR (1995) CD1 recognition by mouse NK1+ T lymphocytes. Science 268:863–865 [DOI] [PubMed] [Google Scholar]
- Bendelac A, Bonneville M, Kearney JF (2001) Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol 1:177–186 [DOI] [PubMed] [Google Scholar]
- Birkinshaw RW, Kjer-Nielsen L, Eckle SB, McCluskey J, Rossjohn J (2014) MAITs, MR1 and vitamin B metabolites. Curr Opin Immunol 26:7–13 [DOI] [PubMed] [Google Scholar]
- Bix M, Locksley RM (1995) Natural T cells. Cells that co-express NKRP-1 and TCR. J Immunol 155:1020–1022 [PubMed] [Google Scholar]
- Bobryshev YV, Lord RS (2005) Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J Histochem Cytochem 53:781–785 [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB (2004) CD1: antigen presentation and T cell function. Annu Rev Immunol 22:817–890 [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB (2010) How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol 22:79–86 [DOI] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB (2003) Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol 4:1230–1237 [DOI] [PubMed] [Google Scholar]
- Brossay L, Tangri S, Bix M, Cardell S, Locksley R, Kronenberg M (1998) Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J Immunol 160:3681–3688 [PubMed] [Google Scholar]
- Brown DR, Fowell DJ, Corry DB, Wynn TA, Moskowitz NH, Cheever AW, Locksley RM, Reiner SL (1996) Beta 2-microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J Exp Med 184:1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, Bosia A, Marchisio PC, Mantovani A (1991) In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest 87:986–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Hsu YH, Li S, Woods VL Jr, Dennis EA (2013) Structural basis of specific interactions of Lp-PLA2 with HDL revealed by hydrogen deuterium exchange mass spectrometry. J Lipid Res 54:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D (1995) CD1-restricted CD4+ T cells in major histocompatibility complex class II deficient mice. J Exp Med 182:993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoto K, Guo T, Imataki O, Tanaka M, Nakatsugawa M, Ochi T, Yamashita Y, Saito AM, Saito TI, Butler MO, Hirano N (2016) CDR3beta sequence motifs regulate autoreactivity of human invariant NKT cell receptors. J Autoimmun 68:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WL, Pejnovic N, Hamilton H, Liew TV, Popadic D, Poggi A, Khan SM (2005) Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells. Circ Res 96: 675–683 [DOI] [PubMed] [Google Scholar]
- Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV (2008) Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood 112:1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Paul WE (1997) Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN- gamma upon activation by anti-CD3 or CD1. J Immunol 159:2240–2249 [PubMed] [Google Scholar]
- Chiu YH, Jayawardena J, Weiss A, Lee D, Park SH, Dautry-Varsat A, Bendelac A (1999) Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med 189:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Hirata K, Ishida T, Quertermous T, Cooper AD (2002) Endothelial lipase: a new lipase on the block. J Lipid Res 43:1763–1769 [DOI] [PubMed] [Google Scholar]
- Christiansen D, Milland J, Mouhtouris E, Vaughan H, Pellicci DG, McConville MJ, Godfrey DI, Sandrin MS (2008) Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol 6:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NR, Garg S, Brenner MB (2009) Antigen presentation by CD1 Lipids, T Cells, and NKT Cells in microbial immunity. Adv Immunol 102:1–94 [DOI] [PubMed] [Google Scholar]
- Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI (2008) Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A 105:11287–11292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Fox L, Tian R, Bardet W, Skaley M, Mojsilovic D, Gumperz J, Hildebrand W (2009) Determination of cellular lipids bound to human CD1d molecules. PLoS One 4:e5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset M, Brossard N, Polette A, Lagarde M (2000) Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J 345(Pt 1):61–67 [PMC free article] [PubMed] [Google Scholar]
- da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL (2014) Novel insights on interactions between folate and lipid metabolism. Biofactors 40:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, Berg T, Paoletti S, Maitre B, Mourey L, Salamero J, Cazenave JP, Hanau D, Mori L, Puzo G, De Libero G (2005) Assistance of microbial glycolipid antigen processing by CD1e. Science 310:1321–1324 [DOI] [PubMed] [Google Scholar]
- Exley M, Garcia J, Balk SP, Porcelli S (1997) Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med 186:109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley MA, Tahir SM, Cheng O, Shaulov A, Joyce R, Avigan D, Sackstein R, Balk SP (2001) A major fraction of human bone marrow lymphocytes are Th2-like CD1d- reactive T cells that can suppress mixed lymphocyte responses. J Immunol 167:5531–5534 [DOI] [PubMed] [Google Scholar]
- Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, Berger J, Xia C, Mori L, De Libero G (2012) Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol 13:474–480 [DOI] [PubMed] [Google Scholar]
- Field JJ, Nathan DG, Linden J (2011) Targeting iNKT cells for the treatment of sickle cell disease. Clin Immunol 140:177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L, Trott DL, Ndonye RM, Veerapen N, Besra GS, Howell AR, Cook ME, Adams EJ, Hildebrand WH, Gumperz JE (2009) Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol 7:e1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freigang S, Zadorozhny V, McKinney MK, Krebs P, Herro R, Pawlak J, Kain L, Schrantz N, Masuda K, Liu Y, Savage PB, Bendelac A, Cravatt BF, Teyton L (2010) Fatty acid amide hydrolase shapes NKT cell responses by influencing the serum transport of lipid antigen in mice. J Clin Invest 120:1873–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294:1871–1875 [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR (2005) Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 3:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt T, Ley K (2015) Monocyte trafficking across the vessel wall. Cardiovasc Res 107:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Nagahira K, Fukuda Y, Nishimura T (2009) Murine NKT cells produce Th17 cytokine interleukin-22. Cell Immunol 254:81–84 [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Brenner MB (2001) CD1-specific T cells in microbial immunity. Curr Opin Immunol 13:471–478 [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM (2000) Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12:211–221 [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Miyake S, Yamamura T, Brenner MB (2002) Functionally distinct subsets of CD1d-restricted natural killer T Cells revealed by CD1d tetramer staining. J Exp Med 195:625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Chen X, Keaton JM, Reddington F, Besra GS, Gumperz JE (2007) NKT cells direct monocytes into a DC differentiation pathway. J Leukoc Biol 81:1224–1235 [DOI] [PubMed] [Google Scholar]
- Kain L, Webb B, Anderson BL, Deng S, Holt M, Costanzo A, Zhao M, Self K, Teyton A, Everett C, Kronenberg M, Zajonc DM, Bendelac A, Savage PB, Teyton L (2014) The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity 41:543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain L, Costanzo A, Webb B, Holt M, Bendelac A, Savage PB, Teyton L (2015) Endogenous ligands of natural killer T cells are alpha-linked glycosylceramides. Mol Immunol 68:94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M (1997) CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278:1626–1629 [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434:520–525 [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M (2006) Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol 7: 978–986 [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491: 717–723 [DOI] [PubMed] [Google Scholar]
- Koseki H, Imai K, Nakayama F, Sado T, Moriwaki K, Taniguchi M (1990) Homogenous junctional sequence of the V14+ T-cell antigen receptor alpha chain expanded in unprimed mice. Proc Natl Acad Sci U S A 87:5248–5252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H, Asano H, Inaba T, Miyashita N, Moriwaki K, Lindahl KF, Mizutani Y, Imai K, Taniguchi M (1991) Dominant expression of a distinctive V14+ T-cell antigen receptor alpha chain in mice. Proc Natl Acad Sci U S A 88:7518–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M, Rudensky A (2005) Regulation of immunity by self-reactive T cells. Nature 435:598–604 [DOI] [PubMed] [Google Scholar]
- Kyriakakis E, Cavallari M, Andert J, Philippova M, Koella C, Bochkov V, Erne P, Wilson SB, Mori L, Biedermann BC, Resink TJ, De Libero G (2010) Invariant natural killer T cells: linking inflammation and neovascularization in human atherosclerosis. Eur J Immunol 40(11):3268–3279 [DOI] [PubMed] [Google Scholar]
- Lantz O, Bendelac A (1994) An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8- T cells in mice and humans. J Exp Med 180:1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P (2010) An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol 11:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA (2013) Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol 14: 1146–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, Hogquist KA (2015) Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity 43:566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kanellakis P, Hosseini H, Cao A, Deswaerte V, Tipping P, Toh BH, Bobik A, Kyaw T (2015a) A CD1d-dependent lipid antagonist to NKT cells ameliorates atherosclerosis in ApoE−/− mice by reducing lesion necrosis and inflammation. Cardiovasc Res 109(2):305–317 [DOI] [PubMed] [Google Scholar]
- Li Y, To K, Kanellakis P, Hosseini H, Deswaerte V, Tipping P, Smyth MJ, Toh BH, Bobik A, Kyaw T (2015b) CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin- and granzyme B-dependent cytotoxicity. Circ Res 116:245–254 [DOI] [PubMed] [Google Scholar]
- Lin G, Field JJ, Yu JC, Ken R, Neuberg D, Nathan DG, Linden J (2013) NF-kappaB is activated in CD4+ iNKT cells by sickle cell disease and mediates rapid induction of adenosine A2A receptors. PLoS One 8:e74664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sagaseta J, Sibener LV, Kung JE, Gumperz J, Adams EJ (2012) Lysophospholipid presentation by CD1d and recognition by a human natural killer T-cell receptor. EMBO J 31:2047–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma AM, Castro CD, Adams EJ (2014) gammadelta T cell surveillance via CD1 molecules. Trends Immunol 35:613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major AS, Wilson MT, McCaleb JL, Ru Su Y, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF (2004) Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 24:2351–2357 [DOI] [PubMed] [Google Scholar]
- Mandel H, Sharf R, Berant M, Wanders RJ, Vreken P, Aviram M (1998) Plasmalogen phospholipids are involved in HDL-mediated cholesterol efflux: insights from investigations with plasmalogen-deficient cells. Biochem Biophys Res Commun 250:369–373 [DOI] [PubMed] [Google Scholar]
- Marathe GK, Pandit C, Lakshmikanth CL, Chaithra VH, Jacob SP, D’Souza CJ (2014) To hydrolyze or not to hydrolyze: the dilemma of platelet-activating factor acetylhydrolase. J Lipid Res 55:1847–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricic I, Girardi E, Zajonc DM, Kumar V (2014) Recognition of lysophosphatidylcholine by type II NKT cells and protection from an inflammatory liver disease. J Immunol 193:4580–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J, Debord KL, Ismail N, Goff RD, Cantu C 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525–529 [DOI] [PubMed] [Google Scholar]
- Melian A, Geng YJ, Sukhova GK, Libby P, Porcelli SA (1999) CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am J Pathol 155:775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira-Teixeira L, Resende M, Coffre M, Devergne O, Herbeuval JP, Hermine O, Schneider E, Rogge L, Ruemmele FM, Dy M, Cordeiro-da-Silva A, Leite-de-Moraes MC (2011) Proinflammatory environment dictates the IL-17-producing capacity of human invariant NKT cells. J Immunol 186:5758–5765 [DOI] [PubMed] [Google Scholar]
- Morita CT, Jin C, Sarikonda G, Wang H (2007) Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 215:59–76 [DOI] [PubMed] [Google Scholar]
- Munn NJ, Arnio E, Liu D, Zoeller RA, Liscum L (2003) Deficiency in ethanolamine plasmalogen leads to altered cholesterol transport. J Lipid Res 44:182–192 [DOI] [PubMed] [Google Scholar]
- Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K, Mishima T, Iwabuchi C, Tanaka S, Bezbradica JS, Nakayama T, Taniguchi M, Miyake S, Yamamura T, Kitabatake A, Joyce S, Van Kaer L, Onoe K (2004) Natural killer T cells accelerate atherogenesis in mice. Blood 104:2051–2059 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R (2014) Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol 34:724–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paduraru C, Bezbradica JS, Kunte A, Kelly R, Shayman JA, Veerapen N, Cox LR, Besra GS, Cresswell P (2013) Role for lysosomal phospholipase A2 in iNKT cell-mediated CD1d recognition. Proc Natl Acad Sci U S A 110:5097–5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, Reantragoon R, Sandoval-Romero ML, Sullivan LC, Brooks AG, Chen Z, Fairlie DP, McCluskey J, Rossjohn J (2013) Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun 4:2142. [DOI] [PubMed] [Google Scholar]
- Porcelli SA, Modlin RL (1999) The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol 17:297–329 [DOI] [PubMed] [Google Scholar]
- Porcelli S, Morita CT, Brenner MB (1992) CD1b restricts the response of human CD4–8- T lymphocytes to a microbial antigen. Nature 360: 593–597 [DOI] [PubMed] [Google Scholar]
- Porcelli S, Gerdes D, Fertig AM, Balk SP (1996) Human T cells expressing an invariant V alpha 24-J alpha Q TCR alpha are CD4- and heterogeneous with respect to TCR beta expression. Hum Immunol 48:63–67 [DOI] [PubMed] [Google Scholar]
- Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ (2007) Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci U S A 104:5977–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puan KJ, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, Samuelson MI, Marker-Hermann E, Pasa-Tolic L, Nieves E, Giner JL, Kuzuyama T, Morita CT (2007) Preferential recognition of a microbial metabolite by human Vgamma2Vdelta2 T cells. Int Immunol 19:657–673 [DOI] [PubMed] [Google Scholar]
- Reantragoon R, Kjer-Nielsen L, Patel O, Chen Z, Illing PT, Bhati M, Kostenko L, Bharadwaj M, Meehan B, Hansen TH, Godfrey DI, Rossjohn J, McCluskey J (2012) Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor. J Exp Med 209:761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DA, Chen HC, Price AJ, Keeble AH, Davey MS, James LC, Eberl M, Trowsdale J (2015) Activation of human gammadelta T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J Immunol 194:2390–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riano F, Karunakaran MM, Starick L, Li J, Scholz CJ, Kunzmann V, Olive D, Amslinger S, Herrmann T (2014) Vgamma9Vdelta2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6. Eur J Immunol 44:2571–2576 [DOI] [PubMed] [Google Scholar]
- Riederer M, Kofeler H, Lechleitner M, Tritscher M, Frank S (2012) Impact of endothelial lipase on cellular lipid composition. Biochim Biophys Acta 1821:1003–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L, Burchat S, Gage J, Hasu M, Thabet M, Willcox L, Ramsamy TA, Whitman SC (2008) Deficiency of invariant Valpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc Res 78:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchi F, Falcone M (2008) Immune regulation by invariant NKT cells in autoimmunity. Front Biosci 13:4827–4837 [DOI] [PubMed] [Google Scholar]
- Sanderson JP, Brennan PJ, Mansour S, Matulis G, Patel O, Lissin N, Godfrey DI, Kawahara K, Zahringer U, Rossjohn J, Brenner MB, Gadola SD (2013) CD1d protein structure determines species-selective antigenicity of isoglobotrihexosylceramide (iGb3) to invariant NKT cells. Eur J Immunol 43:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, Breathnach R, Bonneville M, Scotet E, Adams EJ (2014) The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity 40:490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna J, Garcia-Seisdedos D, Alcazar A, Lasuncion MA, Busto R, Pastor O (2015) Quantitative lipidomic analysis of plasma and plasma lipoproteins using MALDI-TOF mass spectrometry. Chem Phys Lipids 189:7–18 [DOI] [PubMed] [Google Scholar]
- Sevastou I, Kaffe E, Mouratis MA, Aidinis V (2013) Lysoglycerophospholipids in chronic inflammatory disorders: the PLA(2)/LPC and ATX/LPA axes. Biochim Biophys Acta 1831:42–60 [DOI] [PubMed] [Google Scholar]
- Silva IT, Mello AP, Damasceno NR (2011) Antioxidant and inflammatory aspects of lipoprotein-associated phospholipase A(2) (Lp-PLA(2)): a review. Lipids Health Dis 10:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley ST, Kaplan MH, Grusby MJ (1997) Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275:977–979 [DOI] [PubMed] [Google Scholar]
- Snyder F (1988) Metabolism of platelet activating factor and related ether lipids: enzymatic pathways, subcellular sites, regulation, and membrane processing. Prog Clin Biol Res 282:57–72 [PubMed] [Google Scholar]
- Snyder F, Fitzgerald V, Blank ML (1996) Biosynthesis of platelet-activating factor and enzyme inhibitors. Adv Exp Med and Biol 416:5–10 [DOI] [PubMed] [Google Scholar]
- Spada FM, Koezuka Y, Porcelli SA (1998) CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med 188:1529–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafforini DM, Tjoelker LW, McCormick SP, Vaitkus D, McIntyre TM, Gray PW, Young SG, Prescott SM (1999) Molecular basis of the interaction between plasma platelet-activating factor acetylhydrolase and low density lipoprotein. J Biol Chem 274: 7018–7024 [DOI] [PubMed] [Google Scholar]
- Stanic AK, Shashidharamurthy R, Bezbradica JS, Matsuki N, Yoshimura Y, Miyake S, Choi EY, Schell TD, Van Kaer L, Tevethia SS, Roopenian DC, Yamamura T, Joyce S (2003) Another view of T cell antigen recognition: cooperative engagement of glycolipid antigens by Va14Ja18 natural TCR. J Immunol 171:4539–4551 [DOI] [PubMed] [Google Scholar]
- Steinbrecher UP, Pritchard PH (1989) Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J Lipid Res 30:305–315 [PubMed] [Google Scholar]
- Subramanian S, Turner MS, Ding Y, Goodspeed L, Wang S, Buckner JH, O’Brien K, Getz GS, Reardon CA, Chait A (2013) Increased levels of invariant natural killer T lymphocytes worsen metabolic abnormalities and atherosclerosis in obese mice. J Lipid Res 54:2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR (1995) Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 375:155–158 [DOI] [PubMed] [Google Scholar]
- Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A (2011) PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med 208:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K, Agrotis A, Besra G, Bobik A, Toh BH (2009) NKT cell subsets mediate differential proatherogenic effects in ApoE−/− mice. Arterioscler Thromb Vasc Biol 29:671–677 [DOI] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422:164–169 [DOI] [PubMed] [Google Scholar]
- Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP (2004) CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med 199:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupin E, Kinjo Y, Kronenberg M (2007) The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 5:405–417 [DOI] [PubMed] [Google Scholar]
- van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, Besra GS, Kent SC, Moody DB, Brenner MB (2005) Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 437:906–910 [DOI] [PubMed] [Google Scholar]
- Van Rhijn I, Moody DB (2015) Donor unrestricted T cells: a shared human T cell response. J Immunol 195:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB (2015) Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol 15:643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rhijn I, van Berlo T, Hilmenyuk T, Cheng TY, Wolf BJ, Tatituri RV, Uldrich AP, Napolitani G, Cerundolo V, Altman JD, Willemsen P, Huang S, Rossjohn J, Besra GS, Brenner MB, Godfrey DI, Moody DB (2016) Human autoreactive T cells recognize CD1b and phospholipids. Proc Natl Acad Sci U S A 113:380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderLaan PA, Reardon CA, Sagiv Y, Blachowicz L, Lukens J, Nissenbaum M, Wang CR, Getz GS (2007) Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am J Pathol 170:1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, Beddoe T, Theodossis A, Williams NK, Gostick E, Price DA, Soudamini DU, Voon KK, Olivo M, Rossjohn J, Mori L, De Libero G (2013) Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat Immunol 14:908–916 [DOI] [PubMed] [Google Scholar]
- Vincent MS, Xiong X, Grant EP, Peng W, Brenner MB (2005) CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J Immunol 175:6344–6351 [DOI] [PubMed] [Google Scholar]
- Voloshyna I, Littlefield MJ, Reiss AB (2014) Atherosclerosis and interferon-gamma: new insights and therapeutic targets. Trends Cardiovasc Med 24:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, Linden J (2009) NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood 114:667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Morita CT (2015) Sensor function for butyrophilin 3A1 in prenyl pyrophosphate stimulation of human Vgamma2Vdelta2 T cells. J Immunol 195:4583–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Rodenkirch L, Simonson W, Wernimont S, Ndonye RM, Veerapen N, Gibson D, Howell AR, Besra GS, Painter GF, Huttenlocher A, Gumperz JE (2008) Natural killer T-cell autoreactivity leads to a specialized activation state. Blood 112: 4128–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sarikonda G, Puan KJ, Tanaka Y, Feng J, Giner JL, Cao R, Monkkonen J, Oldfield E, Morita CT (2011) Indirect stimulation of human Vgamma2Vdelta2 T cells through alterations in isoprenoid metabolism. J Immunol 187:5099–5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bishop KA, Hegde S, Rodenkirch LA, Pike JW, Gumperz JE (2012) Human invariant natural killer T cells acquire transient innate responsiveness via histone H4 acetylation induced by weak TCR stimulation. J Exp Med 209:987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Henry O, Distefano MD, Wang YC, Raikkonen J, Monkkonen J, Tanaka Y, Morita CT (2013) Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vgamma2Vdelta2 T cells. J Immunol 191:1029–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Erl W, Weber KS, Weber PC (1999) Effects of oxidized low density lipoprotein, lipid mediators and statins on vascular cell interactions. Clin Chem Lab Med 37:243–251 [DOI] [PubMed] [Google Scholar]
- Wong CH, Kubes P (2013) Imaging natural killer T cells in action. Immunol Cell Biol 91:304–310 [DOI] [PubMed] [Google Scholar]
- Wu L, Van Kaer L (2009) Natural killer T cells and autoimmune disease. Curr Mol Med 9:4–14 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE (1995) Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science 270:1845–1847 [DOI] [PubMed] [Google Scholar]
- Young DC, Moody DB (2006) T-cell recognition of glycolipids presented by CD1 proteins. Glycobiology 16:103R–112R [DOI] [PubMed] [Google Scholar]
- Yuan W, Kang SJ, Evans JE, Cresswell P (2009) Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol 182:4784–4791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc DM, Kronenberg M (2009) Carbohydrate specificity of the recognition of diverse glycolipids by natural killer T cells. Immunol Rev 230:188–200 [DOI] [PubMed] [Google Scholar]
- Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L (2008) Crystal structures of mouse CD1d-iGb3 complex and its cognate Valpha14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol 377:1104–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng MY, Pham D, Bagaitkar J, Liu J, Otero K, Shan M, Wynn TA, Brombacher F, Brutkiewicz RR, Kaplan MH, Dinauer MC (2013) An efferocytosis-induced, IL-4-dependent macrophage-iNKT cell circuit suppresses sterile inflammation and is defective in murine CGD. Blood 121:3473–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A (2004) Lysosomal glycosphingolipid recognition by NKT cells. Science 306:1786–1789 [DOI] [PubMed] [Google Scholar]


