Abstract
Objective
To evaluate the association between the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis and muscle density in children and adolescents of short stature.
Methods
Participants were children and adolescents of short stature hospitalized in the Affiliated Hospital of Jining Medical University between January 2020 and June 2021. All participants had CT scan images available. We performed an analysis of the images to calculate the muscle density or skeletal muscle attenuation (SMA), skeletal muscle index (SMI), and fat mass index (FMI). Bioelectrical impedance analysis (BIA) was used to ensure that chest CT is a credible way of evaluating body composition.
Results
A total of 297 subjects were included with the mean age of 10.00 ± 3.42 years, mean height standard deviation score (SDS) of -2.51 ± 0.53, and mean IGF-1 SDS of -0.60 ± 1.07. The areas of muscle and fat tissues at the fourth thoracic vertebra level in the CT images showed strong correlation with the total weights of the participants (R2 = 0.884 and 0.897, respectively). The peak of GH was negatively associated with FMI (r = - 0.323, P <.01) and IGF-1 SDS was positively associated with SMI (r = 0.303, P <.01). Both the peak GH and IGF-1 SDS were positively associated with SMA (r = 0.244, P <.01 and r = 0.165, P <.05, respectively). Multiple stepwise linear regression analysis demonstrated that the GH peak was the predictor of FMI (β = - 0.210, P < .01), the IGF-1 SDS was the predictor of SMI (β = 0.224, P < .01), and both the peak GH and IGF-1 SDS were predictors of SMA (β = 0.180, P < .01 and β = 0.222, P < .01).
Conclusions
A chest CT scan is a credible method of evaluating body composition in children and adolescents of short stature. In these patients, peak GH and IGF-1 SDS are independent predictors of muscle density and the GF/IGF-1 axis may regulate body composition through complex mechanisms.
Keywords: short stature, muscle density, GH/IGF-1 axis, body composition, GDDSD study
Introduction
Growth hormone (GH) promotes linear growth and plays key role in regulating muscle development and metabolism. Insulin-like growth factor-1 (IGF-1) is the major mediator by which GH elicits skeletal muscle cell proliferation and myocyte differentiation (1, 2). Children and adolescents of short stature often have increased fat mass and reduced lean mass and muscle strength. This phenomenon is more distinct in those with severe growth hormone deficiency (GHD) (3–5). Treatment with GH can increase muscle mass and strength and decrease fat tissue percentage. Discontinuing it leads to a reversal of these effects (4, 6–10).
Muscle density is an important parameter of muscle health and is emerging as a predictive factor for various metabolic diseases. In adults, low muscle density is associated with a high risk of diabetes, cardiovascular diseases, bone fractures, and worse outcomes in patients with cancer and other critical illnesses (11–18). Several studies have demonstrated that muscle density is a predictor of bone density, bone strength, and cardio-metabolic risk in children and adolescents (19–21). Currently, computed tomography (CT) is the gold standard for investigating qualitative changes in muscles. Low muscle attenuation indicates a high proportion of myosteatosis (intermuscular and intramuscular fat infiltration); whereas high muscle attenuation indicates low muscle fat infiltration (high muscle density) (16, 22, 23).
Although previous studies have reported that GH plays an important role in maintaining muscle mass, none have investigated the role of GH on muscle density. In the past two years, a chest CT scan was performed on some children and adolescents of short stature admitted to our hospital during the COVID-19 pandemic. We performed this retrospective study to evaluate the relationship between the GH/IGF-1 axis and muscle density in children and adolescents of short stature. The areas of skeletal muscle and fat at the fourth thoracic vertebra (T4), assessed by CT, and the total weight assessed by bioelectrical impedance analysis (BIA) were correlated to ensure chest CT was a credible method of evaluating body composition.
Methods
Study Patients
All the subjects enrolled were in the GDDSD study (http://www.chictr.org.cn, ChiCTR1900026510), an ongoing prospective, observational, open cohort study that is evaluating the etiology of growth and development diseases and the long-term safety and effectiveness of growth hormone therapy in a real-life clinical setting (24). Children and adolescents of short stature in the study were those hospitalized between January 2020 and June 2021 in the Department of Endocrinology of the Affiliated Hospital of Jining Medical University and had a chest CT scan done. Short stature is defined as a condition in which the individual’s height is two standard deviations (SD) or more below the population mean for the relevant age and gender (25). The exclusion criteria were as follows: (1) patients missing the values of IGF-1 and GH stimulation test; (2) patients with chronic disease, malignant tumors, and abnormal thyroid function; and (3) patients with conditions such as skeletal dysplasia, achondroplasia, and disorders of sex development. Approval was obtained from the Ethics Committee of the Affiliated Hospital of Jining Medical University and informed consent forms were signed by all the participants’ parents.
Body Composition Measurements
Two authors of this study identified axial CT images at the T4 level and used them to calculate the skeletal muscle area, subcutaneous fat area, and mean skeletal muscle attenuation (SMA). The Slice-O-Matic software (V.5.0, TomoVision, Montreal, Quebec, Canada) was used in this analysis and the attenuation threshold was set to −29 to 150 Hounsfield units (HU) for skeletal muscle, and −190 to −30 HU for subcutaneous adipose tissue. Each type of tissue found in the T4 CT images was shaded with a different color that corresponded to these thresholds. The T4 cross-sectional skeletal muscle area (T4MA) and subcutaneous fat area (T4FA) were recorded in cm2 and the SMA in mean HU. The skeletal muscle index (SMI) and fat mass index (FMI) were calculated by dividing the skeletal muscle and subcutaneous fat area in cm2 by height in m2. The total muscle mass (TMW) and total fat tissue mass (TFW) were measured using BIA with patients in a fasting state.
Laboratory Measurements
Overnight fasting blood samples were collected from all participants and laboratory parameters were measured using methods described in a study we did previously (24). The biochemical and immune indices used in this study include GH, IGF-1, IGF-binding protein-3 (IGFBP-3), hemoglobin (Hb), alanine aminotransferase (ALT), albumin (ALB), creatinine (Cr), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), blood calcium (Ca), blood phosphate (P), free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The IGF-1 SD score (SDS) was calculated using the reference values in healthy children of the same age and sex (26). Two of three GH stimulating tests were performed to evaluate the peak level of GH (levodopa, 500 mg for those ≥ 30 kg, 250 mg for those < 30 kg and ≥ 15 kg, and 125 mg for those < 15 kg; insulin, 0.1-0.15 U/kg; and arginine, 0.5 mg/kg). Blood samples were collected at 0, 30, 60, 90, and 120 minutes to obtain serum GH concentrations at each of these points.
Statistical Analysis
Continuous variables were summarized using the median and IQR for non-normally distributed data and the mean ± SD for normally distributed data. Categorical variables were summarized as the frequency count in percentage. Correlations between variables were assessed by Pearson’s correlation coefficient. Multiple stepwise linear regression analysis was used to identify independent factors associated with muscle density. Statistical analysis was performed using SPSS software (26.0; IBM, Armonk, NY). A P value less than 0.05 was considered statistically significant.
Results
A total of 297 eligible participants (189 males and 108 females) were included. The mean age was 10.00 ± 3.42 years (ranging from 4 to 16 years). Their mean height SDS was −2.51 ± 0.53 and the mean IGF-1 SDS was -0.60 ± 1.07. The body compositions measured by BIA and chest CT and other parameters are summarized in Table 1.
Table 1.
Characteristics of patients included in this study.
| Characteristic | Patients | Value | Characteristic | Patients | Value |
|---|---|---|---|---|---|
| Age (years) | 297 | 10.00 ± 3.42 | Hb (g/L) | 296 | 131.30 ± 11.04 |
| Bone age (years) | 289 | 8.43 ± 3.83 | ALT (U/L) | 296 | 14.70 ± 8.07 |
| Peak of GH (ng/mL) | 297 | 7.29 ± 5.00 | ALB (g/L) | 296 | 46.88 ± 2.86 |
| IGF-1 SDS | 297 | -0.60 ± 1.07 | Cr (umol/L) | 295 | 43.82 ± 11.66 |
| IGFBP-3 (μg/mL) | 293 | 4.55 ± 1.31 | TG (mmol/L) | 282 | 0.68 (0.53,0.91) |
| High SDS | 297 | -2.51 ± 0.53 | TC (mmol/L) | 282 | 3.91 ± 0.75 |
| Weight (kg) | 297 | 27.94 ± 11.91 | HDL (mmol/L) | 282 | 1.52 ± 0.41 |
| BMI SDS | 297 | -0.22 ± 1.53 | LDL (mmol/L) | 282 | 2.21 ± 0.52 |
| SMA (HU) | 297 | 47.54 ± 4.05 | Ca (mmol/L) | 296 | 2.45 ± 0.10 |
| T4MA (cm2) | 297 | 94.85 ± 31.63 | P (mmol/L) | 296 | 1.60 ± 0.16 |
| T4FA (cm2) | 297 | 33.83 (22.57,58.23) | FT3 (pmol/L) | 294 | 6.88 ± 2.26 |
| SMI (cm2/m2) | 297 | 59.03 ± 8.83 | FT4 (pmol/L) | 294 | 17.90 ± 3.01 |
| FMI (cm2/m2) | 297 | 23.99 (15.59,37.22) | TSH (mIU/L) | 294 | 2.66 ± 1.36 |
| TMW (kg) | 205 | 10.88 ± 4.64 | LH (mIU/ml) | 281 | 0.23 (0.01,1.57) |
| TFW (kg) | 205 | 4.90 (2.90,7.95) | FSH (mIU/ml) | 281 | 2.45 (1.05,4.36) |
The Relationship Between CT Scan and BIA in Measuring Body Composition
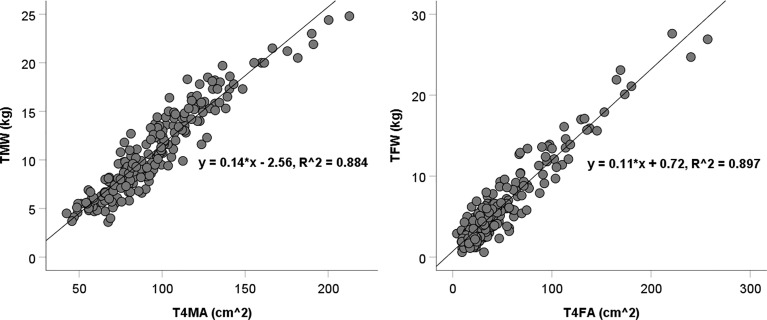
Scatter plots show the T4MA and T4FA assessed by chest CT and TMW and TFW assessed by BIA (Figure 1). The T4MA showed a strong correlation with TMW (TMW = 0.14 T4MA – 2.56; R2 = 0.884; P <.001) and T4FA showed a strong correlation with TFW (TFW = 0.11 T4FA + 0.72; R2 = 0.897; P <.001).
Figure 1.
Scatter diagram of T4MA and T4FA with TMW and TFW. TMW, total muscle weight; TFW, total fat weight; T4MA, T4 muscle area; T4FA, T4 fat area.
The Correlation Between the GH/IGF-1 Axis and CT Scan Body Composition
Male patients had a higher level of SMI (60.48 ± 8.93 and 56.79 ± 8.10, P <.01) compared with female patients, but there were no differences in FMI and SMA. The peak GH was negatively associated with FMI (r = - 0.323, P <.01) and positively associated with SMA (r = 0.244, P <.01). The level of IGF-1 SDS was positively associated with SMI (r = 0.303, P <.01) and SMA (r = 0.165, P <.05). The correlations between body composition and other clinical factors are shown in Table 2.
Table 2.
Correlations between body composition evaluated by T4 CT scan and clinical factors.
| Variables | SMI (cm2/m2) | FMI (cm2/m2) | SMA (HU) |
|---|---|---|---|
| Age (years) | - 0.027 | 0.195* | - 0.229** |
| Bone age (years) | 0.036 | 0.234** | - 0.217** |
| High SDS | 0.028 | - 0.091 | 0.108 |
| Weight (kg) | 0.198* | 0.535** | - 0.338** |
| BMI SDS | 0.293** | 0.563** | - 0.222** |
| Peak of GH (ng/mL) | 0.071 | - 0.323** | 0.244** |
| IGF-1 SDS | 0.303** | 0.069 | 0.165* |
| IGFBP-3 (μg/mL) | 0.054 | 0.132* | - 0.085 |
| Hb (g/L) | 0.216** | 0.098 | 0.075 |
| ALT (U/L) | 0.101 | 0.404** | - 0.222** |
| ALB (g/L) | 0.067 | 0.137* | - 0.060 |
| Cr (μmol/L) | 0.177* | 0.053 | 0.073 |
| TG (mmol/L) | 0.135* | 0.210** | 0.005 |
| TC (mmol/L) | - 0.144* | 0.080 | - 0.129* |
| HDL (mmol/L) | - 0.005 | - 0.076 | 0.002 |
| LDL (mmol/L) | - 0.026 | 0.289** | - 0.114 |
| Ca (mmol/L) | 0.049 | 0.112 | - 0.042 |
| P (mmol/L) | - 0.057 | - 0.043 | 0.078 |
| FT3 (pmol/L) | 0.127* | 0.136* | - 0.041 |
| FT4 (pmol/L) | - 0.013 | - 0.105 | 0.039 |
| TSH (mIU/L) | 0.029 | 0.058 | 0.004 |
| LH (mIU/mL) | 0.055 | 0.124* | - 0.103 |
| FSH (mIU/mL) | 0.008 | 0.214** | - 0.175* |
Correlations are shown with the coefficient r value. * P < 0.05; ** P < 0.01.
Multiple stepwise linear regression analyses of variables related to the SMI, FMI and SMA are listed in Table 3. After adjusting for confounding factors, the peak GH was the predictor of FMI (β = - 0.210, P < .01) and SMA (β = 0.180, P < .01); IGF-1 SDS was the predictor of SMI (β = 0.224, P < 0.01) and SMA (β = 0.222, P < .01).
Table 3.
Multiple stepwise linear regression analysis of factors associated with SMI, FMI and SMA.
| SMI (cm2/m2) | FMI (cm2/m2) | SMA (HU) | |||
|---|---|---|---|---|---|
| Variables | β value | Variables | β value | Variables | β value |
| IGF-1SDS | 0.224** | BMI SDS | 0.403** | Peak of GH (ng/ml) | 0.180** |
| BMI SDS | 0.278** | ALT (U/L) | 0.234** | Age (years) | - 0.264** |
| Gender (male) | 0.210** | Peak of GH (ng/mL) | - 0.210** | IGF-1SDS | 0.222** |
| TC (mmol/L) | - 0.110* | LDL (mmol/L) | 0.203** | BMI SDS | - 0.186** |
| – | – | Bone age (years) | 0.186** | TC (mmol/L) | - 0.132* |
| – | – | FSH (mIU/mL) | 0.109* | ALT (U/L) | - 0.134* |
| - | - | - | - | - | - |
Adopted factors: gender, BMI SDS, IGF-1SDS, Hb, Cr, TG, TC and FT3 for SMI; age, bone age, BMI SDS, the peak of GH, IGFBP-3, ALT, ALB, TG, LDL, FT3, LH and FSH for FMI; age, bone age, BMI SDS, the peak of GH, IGF-1SDS, ALT, TC and FSH for SMA. * P < 0.05; ** P < 0.01.
Discussion
In this study, the peak GH and IGF-1 SDS are positively correlated with muscle density in children and adolescents of short stature. After adjusting for confounding factors, both the peak GH and IGF-1 SDS are independent predictors of muscle density. To the best of our knowledge, this is the first study that demonstrates the relationship between the GH/IGF-1 axis and muscle density.
Several technologies including ultrasonography, dual x-ray absorptiometry, BIA, CT, and magnetic resonance imaging could be used to assess body composition (27). Among these, CT scans quantify bone mineral density, visceral and subcutaneous fat, skeletal muscle, liver fat, and arterial vascular calcification. Thus, they are the most comprehensive modality (28). The predictive value of a chest CT in whole-body composition has been evaluated in healthy adults or patients with cancer. The cross-sectional areas of muscle and fat tissue have shown a moderate correlation with total body weight (29–31). In this study, most of the participants are prepubertal or adolescent, and there is little interference from the abdomen, hip, and limbs. Our research demonstrated that the areas of muscle and fat tissues at the T4 level assessed by chest CT highly correlated with the total weights assessed by BIA (R2 = 0.884 and 0.897, respectively). This provides a possibility of assessing body composition incidentally in some children if chest CT is required for their diagnosis and treatment.
Children and adolescents of short stature, especially those with GHD, often have increased fat mass and reduced lean mass and muscle strength. In 2016, Improda et al. summarized the role of the GH/IGF-1 axis in the muscle and skeletal health of children and adolescents (32). In general, childhood-onset GHD can affect bone and muscle mass and strength, and GH replacement therapy has beneficial effects. Moreover, GH withdrawal at final height can result in reduced bone and muscle mass, potentially leading to increased fracture risk in adulthood (32). Our study also confirmed the association between the GH/IGF-1 axis and body composition. The peak GH is correlated with FMI and IGF-1 SDS is correlated with SMI. This indicates that the GH/IGF-1 axis uses different mechanisms in the regulation of muscle and fat development and metabolism. Unlike skeletal muscle cell proliferation and myocyte differentiation by GH, which are almost entirely mediated by IGF-1, adipose tissue lipolysis appears to be directly mediated via the GH receptor (33–35). The body composition, in turn, also affects the levels of the peak GH and IGF-1. For example, obesity reversibly suppresses GH secretion driven by elevated free fatty acids, whereas IGF-I levels remain normal or elevated due to elevated portal insulin levels (36). Therefore, there are bidirectional associations exist between the GH/IGF-1 axis and body composition.
It is well known that adults of short stature or GHD are at a higher risk of hypertension, dyslipidemia, cardiovascular disease, type 2 diabetes, and fracture (37–42). Coincidentally, individuals with lower muscle attenuation on CT also have a higher risk of these metabolic diseases (15, 16, 43–47). It is possible that patients of short stature already have impaired muscle density in their childhood and the GH/IGF-1 axis plays a critical role in muscle density regulation. Our study demonstrated that both the peak GH and IGF-1 SDS are independent predictors of SMA in children and adolescents of short stature. SMA is a comprehensive marker that determines both SMI and FMI, and the GH/IGF-1 axis may regulate muscle density through complex mechanisms (48, 49).
Our study has several limitations. First, the cross-sectional design of the study does not allow for causal inference and is limited in clarifying the underlying pathophysiological mechanisms involved. Second, to determine whether lower muscle density in childhood can predict higher risks of metabolic diseases, a long-term follow-up is required. Third, some confounding factors that might influence muscle health such as family income, exercise intensity, and dietary habits were not included in the analysis. Fourth, most of the participants enrolled in our study were prepubertal and only SMI showed a gender difference in them. We did not perform subgroup analyses like those done in adult studies. Lastly, our study was conducted in China and the findings may not be readily generalizable in other populations or ethnicities.
In conclusion, this study confirmed the credibility of chest CT in evaluating body composition in children and adolescents of short stature. In these patients, both the peak GH and IGF-1 SDS are independent predictors of muscle density; and the GH/IGF-1 axis may regulate body composition through complex mechanisms.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the Affiliated Hospital of Jining Medical University (2019C003, Jining, China). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
The manuscript was conceived by QY and FL, with manuscript questions and analytic plan designed by GY, QY, BB, and FL. FL wrote the manuscript, interpreted the data, critically reviewed and revised the manuscript. GY, QY, MZ, and BB contributed to writing, data analysis, data interpretation, critical review and revision. YL, YZ, SC, and DH contributed to data interpretation, critical review and revision. All authors had access to the data and all authors agreed to submit the final manuscript. FL was supported by the Jining Key Research and Development Projects. GY, QY, and FL are the guarantors of this work and as such had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The study was supported by the Jining Key Research and Development Projects (2021YXNS073).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate the linguistic assistance provided by TopEdit (www.topeditsci.com) during the preparation of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.920200/full#supplementary-material
References
- 1. Chia DJ. Minireview: Mechanisms of Growth Hormone-Mediated Gene Regulation. Mol Endocrinol (Baltimore Md) (2014) 28(7):1012–25. doi: 10.1210/me.2014-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woodhouse LJ, Mukherjee A, Shalet SM, Ezzat S. The Influence of Growth Hormone Status on Physical Impairments, Functional Limitations, and Health-Related Quality of Life in Adults. Endocr Rev (2006) 27(3):287–317. doi: 10.1210/er.2004-0022 [DOI] [PubMed] [Google Scholar]
- 3. Ji YT, Li LL, Cai SZ, Shi XY. Body Composition in Preschool Children With Short Stature: A Case-Control Study. BMC Pediatr (2022) 22(1):98. doi: 10.1186/s12887-022-03159-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schweizer R, Martin DD, Haase M, Roth J, Trebar B, Binder G, et al. Similar Effects of Long-Term Exogenous Growth Hormone (GH) on Bone and Muscle Parameters: A pQCT Study of GH-Deficient and Small-for-Gestational-Age (SGA) Children. Bone (2007) 41(5):875–81. doi: 10.1016/j.bone.2007.06.028 [DOI] [PubMed] [Google Scholar]
- 5. Matusik P, Klesiewicz M, Klos K, Stasiulewicz M, Barylak A, Nazarkiewicz P, et al. Baseline Body Composition in Prepubertal Short Stature Children With Severe and Moderate Growth Hormone Deficiency. Int J Endocrinol (2016) 2016:4563721. doi: 10.1155/2016/4563721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Attanasio AF, Shavrikova E, Blum WF, Cromer M, Child CJ, Paskova M, et al. Continued Growth Hormone (GH) Treatment After Final Height Is Necessary to Complete Somatic Development in Childhood-Onset GH-Deficient Patients. J Clin Endocrinol Metab (2004) 89(10):4857–62. doi: 10.1210/jc.2004-0551 [DOI] [PubMed] [Google Scholar]
- 7. Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB. Long-Term Growth Hormone Therapy Changes the Natural History of Body Composition and Motor Function in Children With Prader-Willi Syndrome. J Clin Endocrinol Metab (2010) 95(3):1131–6. doi: 10.1210/jc.2009-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leger J, Carel C, Legrand I, Paulsen A, Hassan M, Czernichow P. Magnetic Resonance Imaging Evaluation of Adipose Tissue and Muscle Tissue Mass in Children With Growth Hormone (GH) Deficiency, Turner's Syndrome, and Intrauterine Growth Retardation During the First Year of Treatment With GH. J Clin Endocrinol Metab (1994) 78(4):904–9. doi: 10.1210/jcem.78.4.8157719 [DOI] [PubMed] [Google Scholar]
- 9. Rutherford OM, Jones DA, Round JM, Buchanan CR, Preece MA. Changes in Skeletal Muscle and Body Composition After Discontinuation of Growth Hormone Treatment in Growth Hormone Deficient Young Adults. Clin Endocrinol (1991) 34(6):469–75. doi: 10.1111/j.1365-2265.1991.tb00327.x [DOI] [PubMed] [Google Scholar]
- 10. Schweizer R, Martin DD, Schönau E, Ranke MB. Muscle Function Improves During Growth Hormone Therapy in Short Children Born Small for Gestational Age: Results of a Peripheral Quantitative Computed Tomography Study on Body Composition. J Clin Endocrinol Metab (2008) 93(8):2978–83. doi: 10.1210/jc.2007-2600 [DOI] [PubMed] [Google Scholar]
- 11. Aleixo GFP, Williams GR, Nyrop KA, Muss HB, Shachar SS. Muscle Composition and Outcomes in Patients With Breast Cancer: Meta-Analysis and Systematic Review. Breast Cancer Res Treat (2019) 177(3):569–79. doi: 10.1007/s10549-019-05352-3 [DOI] [PubMed] [Google Scholar]
- 12. Sun C, Anraku M, Kawahara T, Karasaki T, Konoeda C, Kitano K, et al. Combination of Skeletal Muscle Mass and Density Predicts Postoperative Complications and Survival of Patients With Non-Small Cell Lung Cancer. Ann Surg Oncol (2022) 29(3):1816–24. doi: 10.1245/s10434-021-11024-8 [DOI] [PubMed] [Google Scholar]
- 13. van Baar H, Beijer S, Bours MJL, Weijenberg MP, van Zutphen M, van Duijnhoven FJB, et al. Low Radiographic Muscle Density is Associated With Lower Overall and Disease-Free Survival in Early-Stage Colorectal Cancer Patients. J Cancer Res Clin Oncol (2018) 144(11):2139–47. doi: 10.1007/s00432-018-2736-z [DOI] [PubMed] [Google Scholar]
- 14. Li X, Zhang Y, Xie Y, Lu R, Tao H, Chen S. Correlation Between Bone Mineral Density (BMD) and Paraspinal Muscle Fat Infiltration Based on QCT: A Cross-Sectional Study. Calcified Tissue Int (2022) 110(6):666–73. doi: 10.1007/s00223-022-00944-6 [DOI] [PubMed] [Google Scholar]
- 15. Tanaka M, Okada H, Hashimoto Y, Kumagai M, Nishimura H, Fukui M. Low-Attenuation Muscle is a Predictor of Diabetes Mellitus: A Population-Based Cohort Study. Nutr (Burbank Los Angeles County Calif) (2020) 74:110752. doi: 10.1016/j.nut.2020.110752 [DOI] [PubMed] [Google Scholar]
- 16. Lee MJ, Kim HK, Kim EH, Bae SJ, Kim KW, Kim MJ, et al. Association Between Muscle Quality Measured by Abdominal Computed Tomography and Subclinical Coronary Atherosclerosis. Arteriosc Thromb Vasc Biol (2021) 41(2):e128–40. doi: 10.1161/atvbaha.120.315054 [DOI] [PubMed] [Google Scholar]
- 17. Puthucheary ZA, Phadke R, Rawal J, McPhail MJ, Sidhu PS, Rowlerson A, et al. Qualitative Ultrasound in Acute Critical Illness Muscle Wasting. Crit Care Med (2015) 43(8):1603–11. doi: 10.1097/ccm.0000000000001016 [DOI] [PubMed] [Google Scholar]
- 18. Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Twisk JW, Oudemans-van Straaten HM, et al. Skeletal Muscle Quality as Assessed by CT-Derived Skeletal Muscle Density is Associated With 6-Month Mortality in Mechanically Ventilated Critically Ill Patients. Crit Care (London England) (2016) 20(1):386. doi: 10.1186/s13054-016-1563-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyd-Clark LC, Briggs CA, Galea MP. Muscle Spindle Distribution, Morphology, and Density in Longus Colli and Multifidus Muscles of the Cervical Spine. Spine (2002) 27(7):694–701. doi: 10.1097/00007632-200204010-00005 [DOI] [PubMed] [Google Scholar]
- 20. Farr JN, Laddu DR, Blew RM, Lee VR, Going SB. Effects of Physical Activity and Muscle Quality on Bone Development in Girls. Med Sci Sports Exercise (2013) 45(12):2332–40. doi: 10.1249/MSS.0b013e31829c32fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laddu DR, Farr JN, Lee VR, Blew RM, Stump C, Houtkooper L, et al. Muscle Density Predicts Changes in Bone Density and Strength: A Prospective Study in Girls. J Musculoskeletal Neuronal Interactions (2014) 14(2):195–204. [PMC free article] [PubMed] [Google Scholar]
- 22. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of Skeletal Muscle Radiation Attenuation and Basis of its Biological Variation. Acta Physiol (Oxford England) (2014) 210(3):489–97. doi: 10.1111/apha.12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee K, Shin Y, Huh J, Sung YS, Lee IS, Yoon KH, et al. Recent Issues on Body Composition Imaging for Sarcopenia Evaluation. Korean J Radiol (2019) 20(2):205–17. doi: 10.3348/kjr.2018.0479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Q, Zhang M, Chu Y, Sun H, Pan H, Ban B. A Retrospective Analysis of Patients With Short Stature in Eastern China Between 2013 and 2019. BioMed Res Int (2021), 6640026. doi: 10.1155/2021/6640026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rogol AD, Hayden GF. Etiologies and Early Diagnosis of Short Stature and Growth Failure in Children and Adolescents. J Pediatr (2014) 164(5 Suppl):S1–14.e6. doi: 10.1016/j.jpeds.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 26. Isojima T, Shimatsu A, Yokoya S, Chihara K, Tanaka T, Hizuka N, et al. Standardized Centile Curves and Reference Intervals of Serum Insulin-Like Growth Factor-I (IGF-I) Levels in a Normal Japanese Population Using the LMS Method. Endocr J (2012) 59(9):771–80. doi: 10.1507/endocrj.ej12-0110 [DOI] [PubMed] [Google Scholar]
- 27. Ceniccola GD, Castro MG, Piovacari SMF, Horie LM, Corrêa FG, Barrere APN, et al. Current Technologies in Body Composition Assessment: Advantages and Disadvantages. Nutr (Burbank Los Angeles County Calif) (2019) 62:25–31. doi: 10.1016/j.nut.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 28. Pickhardt PJ, Summers RM, Garrett JW. Automated CT-Based Body Composition Analysis: A Golden Opportunity. Korean J Radiol (2021) 22(12):1934–7. doi: 10.3348/kjr.2021.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halpenny DF, Goncalves M, Schwitzer E, Golia Pernicka J, Jackson J, Gandelman S, et al. Computed Tomography-Derived Assessments of Regional Muscle Volume: Validating Their Use as Predictors of Whole Body Muscle Volume in Cancer Patients. Br J Radiol (2018) 91(1092):20180451. doi: 10.1259/bjr.20180451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim YS, Kim EY, Kang SM, Ahn HK, Kim HS. Single Cross-Sectional Area of Pectoralis Muscle by Computed Tomography - Correlation With Bioelectrical Impedance Based Skeletal Muscle Mass in Healthy Subjects. Clin Physiol Funct Imag (2017) 37(5):507–11. doi: 10.1111/cpf.12333 [DOI] [PubMed] [Google Scholar]
- 31. Mathur S, Rozenberg D, Verweel L, Orsso CE, Singer LG. Chest Computed Tomography is a Valid Measure of Body Composition in Individuals With Advanced Lung Disease. Clin Physiol Funct Imag (2020) 40(5):360–8. doi: 10.1111/cpf.12652 [DOI] [PubMed] [Google Scholar]
- 32. Improda N, Capalbo D, Esposito A, Salerno M. Muscle and Skeletal Health in Children and Adolescents With GH Deficiency. Best Pract Res Clin Endocrinol Metab (2016) 30(6):771–83. doi: 10.1016/j.beem.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 33. Flint DJ, Binart N, Kopchick J, Kelly P. Effects of Growth Hormone and Prolactin on Adipose Tissue Development and Function. Pituitary (2003) 6(2):97–102. doi: 10.1023/b:pitu.0000004800.57449.67 [DOI] [PubMed] [Google Scholar]
- 34. Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The Effects of Growth Hormone on Adipose Tissue: Old Observations, New Mechanisms. Nat Rev Endocrinol (2020) 16(3):135–46. doi: 10.1038/s41574-019-0280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chaves VE, Júnior FM, Bertolini GL. The Metabolic Effects of Growth Hormone in Adipose Tissue. Endocrine (2013) 44(2):293–302. doi: 10.1007/s12020-013-9904-3 [DOI] [PubMed] [Google Scholar]
- 36. Hjelholt A, Høgild M, Bak AM, Arlien-Søborg MC, Bæk A, Jessen N, et al. Growth Hormone and Obesity. Endocrinol Metab Clin North Am (2020) 49(2):239–50. doi: 10.1016/j.ecl.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 37. Wittenbecher C, Kuxhaus O, Boeing H, Stefan N, Schulze MB. Associations of Short Stature and Components of Height With Incidence of Type 2 Diabetes: Mediating Effects of Cardiometabolic Risk Factors. Diabetologia (2019) 62(12):2211–21. doi: 10.1007/s00125-019-04978-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vangipurapu J, Stancáková A, Jauhiainen R, Kuusisto J, Laakso M. Short Adult Stature Predicts Impaired β-Cell Function, Insulin Resistance, Glycemia, and Type 2 Diabetes in Finnish Men. J Clin Endocrinol Metab (2017) 102(2):443–50. doi: 10.1210/jc.2016-2933 [DOI] [PubMed] [Google Scholar]
- 39. Paajanen TA, Oksala NK, Kuukasjärvi P, Karhunen PJ. Short Stature is Associated With Coronary Heart Disease: A Systematic Review of the Literature and a Meta-Analysis. Eur Heart J (2010) 31(14):1802–9. doi: 10.1093/eurheartj/ehq155 [DOI] [PubMed] [Google Scholar]
- 40. Zhao Q, Chu Y, Pan H, Zhang M, Ban B. Association Between Triglyceride Glucose Index and Peak Growth Hormone in Children With Short Stature. Sci Rep (2021) 11(1):1969. doi: 10.1038/s41598-021-81564-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao Q, Jiang Y, Zhang M, Chu Y, Ji B, Pan H, et al. Low-Density Lipoprotein Cholesterol Levels are Associated With Insulin-Like Growth Factor-1 in Short-Stature Children and Adolescents: A Cross-Sectional Study. Lipids Health Disease (2019) 18(1):120. doi: 10.1186/s12944-019-1062-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oh NK, Song YM, Kim SH, Park MJ. Short Stature is Associated With Increased Risk of Dyslipidemia in Korean Adolescents and Adults. Sci Rep (2019) 9(1):14090. doi: 10.1038/s41598-019-50524-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ballin M, Nordström P, Niklasson J, Nordström A. Associations of Visceral Adipose Tissue and Skeletal Muscle Density With Incident Stroke, Myocardial Infarction, and All-Cause Mortality in Community-Dwelling 70-Year-Old Individuals: A Prospective Cohort Study. J Am Heart Assoc (2021) 10(9):e020065. doi: 10.1161/jaha.120.020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trikudanathan G, Vantanasiri K, Faizi N, Munigala S, Vanek P, Schat R, et al. Decreased Skeletal Muscle Density is an Independent Predictor of Mortality in Necrotizing Pancreatitis- A Single Tertiary Center Experience in 507 Patients. Pancreatology (2021) 21(6):1146–51. doi: 10.1016/j.pan.2021.05.010 [DOI] [PubMed] [Google Scholar]
- 45. Larsen B, Bellettiere J, Allison M, McClelland RL, Miljkovic I, Vella CA, et al. Muscle Area and Density and Risk of All-Cause Mortality: The Multi-Ethnic Study of Atherosclerosis. Metab: Clin Experiment (2020) 111:154321. doi: 10.1016/j.metabol.2020.154321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yin L, Xu Z, Wang L, Li W, Zhao Y, Su Y, et al. Associations of Muscle Size and Density With Proximal Femur Bone in a Community Dwelling Older Population. Front Endocrinol (2020) 11:503. doi: 10.3389/fendo.2020.00503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang L, Yin L, Zhao Y, Su Y, Sun W, Liu Y, et al. Muscle Density Discriminates Hip Fracture Better Than Computed Tomography X-Ray Absorptiometry Hip Areal Bone Mineral Density. J Cachexia Sarcopenia Muscle (2020) 11(6):1799–812. doi: 10.1002/jcsm.12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang Q, Zhang M, Sun P, Li Y, Xu H, Wang K, et al. Cre/CysC Ratio may Predict Muscle Composition and is Associated With Glucose Disposal Ability and Macrovascular Disease in Patients With Type 2 Diabetes. BMJ Open Diabetes Res Care (2021) 9(2). doi: 10.1136/bmjdrc-2021-002430.Citedin:Pubmed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Longo S, Coratella G, Rampichini S, Borrelli M, Scurati R, Limonta E, et al. Local Fat Content and Muscle Quality Measured by a New Electrical Impedance Myography Device: Correlations With Ultrasound Variables. Eur J Sport Sci (2021) 21(3):388–99. doi: 10.1080/17461391.2020.1751306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.