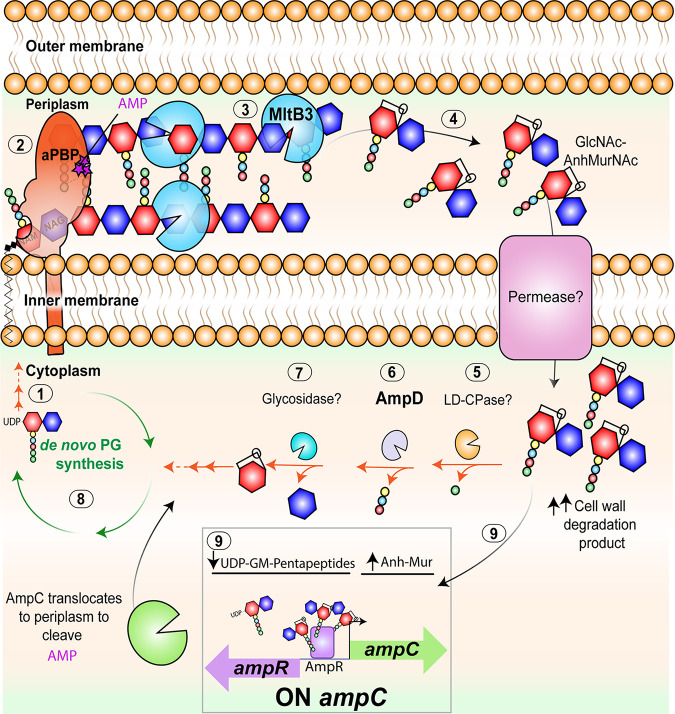
FIG 6.
Working model for A. tumefaciens ampicillin resistance. Bifunctional PBPs extend the cell wall through the transglycosylation (linking of carbohydrates) and transpeptidation (linking of peptide stems) reactions using cytoplasmic precursors (step 1). β-Lactams such as ampicillin (purple stars) target the transpeptidase domain of penicillin-binding proteins (PBPs) (step 2), leading to a block in bacterial cell growth and increased hydrolytic activity by lytic transglycosylases. In A. tumefaciens, inactivation of the lytic transglycosylase MltB3 results in inhibition of β-lactamase derepression and lysis, suggesting that MltB3 is likely required for the generation of cell wall degradation products (step 3) that are transported to the cytoplasm (step 4). In the cytoplasm, hydrolytic enzymes (steps 5 to 7) digest cell wall degradation products and promote PG recycling, enabling de novo PG synthesis (step 8). Similar to treatment with β-lactams, where a block in cell growth leads to an increase in cell wall degradation products (Anh-Mur), inactivation of anhydro amidases such as AmpD (step 6) increases the pool of cell wall degradation products, leading to β-lactam resistance. In A. tumefaciens, inactivation of AmpD leads to derepression of β-lactamases and ampicillin resistance (step 9). Both AmpC, an inducible β-lactamase that is under the transcriptional control of AmpR, and AmpR seem to be responsible for the derepression observed in ΔampD cells. Thus, our working model suggests that upon ampicillin exposure, a block in growth leads to increased activity of MltB3. An increase in cell wall degradation products leads to induction of AmpC expression by AmpR and the presumed translocation of AmpC to the periplasm, resulting in ampicillin resistance.