Figure 1. Activity determination of RhoA (Ras homolog gene family, member A) variants and impact of VEGF (vascular endothelial growth factor) on RhoA activity in human endothelial cells.
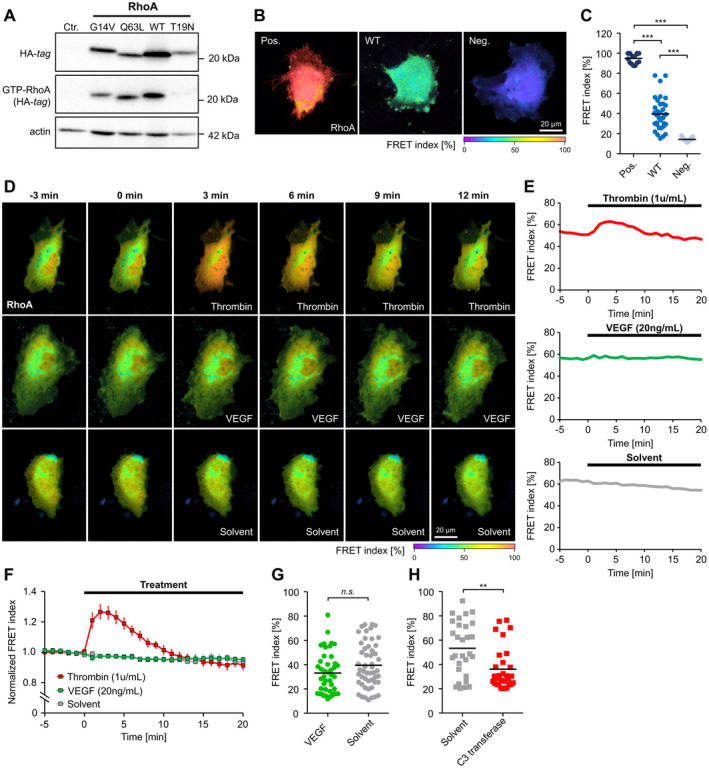
A, Pull down of GTP‐bound RhoA (GTP‐RhoA) derived from constitutively active RhoA (G14V, Q63L), wild‐type (WT) RhoA and dominant‐negative RhoA (T19N) using rhotekin‐coated beads. B and C, Validation of a RhoA Förster resonance energy transfer (FRET) biosensor after transfection of constitutively active (Pos.), WT, and dominant‐negative (Neg.) variants of the sensor in human umbilical vein endothelial cells (HUVEC). B, Representative images of the color‐coded FRET index in HUVEC transfected with the different RhoA variants. C, Statistical analysis shows that the activity of WT‐RhoA is intermediate between the RhoA activity of constitutively active and dominant‐negative controls. ***P<0.001. D, Time‐lapse recordings of HUVEC transfected with the WT RhoA biosensor. The color‐coded FRET index indicates a dynamic change in the RhoA activation state after application of thrombin (1 U/mL), but not after application of human vascular endothelial growth factor (VEGF; 20 ng/mL) or solvent. E, Changes in FRET index over time of single cells shown in (D). F, Average baseline‐normalized FRET index after stimulation of HUVEC with thrombin (n=9), VEGF (n=12), or solvent (n=6). Thrombin induces a transient RhoA activation, whereas no RhoA activation was observed after application of VEGF. G, FRET index of HUVEC measured after long‐term stimulation (18 hours) with VEGF (n=43) or solvent (n=52) indicates no VEGF‐related change in RhoA activity. H, Long‐term application (18 hours) of the RhoA inhibitor C3 transferase (n=30) reduces RhoA activity compared with solvent control (n=29). **P<0.01. Ctr. indicates control‐transduced; HA indicates hemagglutinin; and n.s., not significant.
