Abstract
Background
Renin‐angiotensin aldosterone system (RAAS) inhibitor—COVID‐19 studies, observational in design, appear to use biased methods that can distort the interaction between RAAS inhibitor use and COVID‐19 risk. This study assessed the extent of bias in that research and reevaluated RAAS inhibitor—COVID‐19 associations in studies without critical risk of bias.
Methods and Results
Searches were performed in MEDLINE, EMBASE, and CINAHL databases (December 1, 2019 to October 21, 2021) identifying studies that compared the risk of infection and/or severe COVID‐19 outcomes between those using or not using RAAS inhibitors (ie, angiotensin‐converting enzyme inhibitors or angiotensin II type‐I receptor blockers). Weighted hazard ratios (HR) and 95% CIs were extracted and pooled in fixed‐effects meta‐analyses, only from studies without critical risk of bias that assessed severe COVID‐19 outcomes. Of 169 relevant studies, 164 had critical risks of bias and were excluded. Ultimately, only two studies presented data relevant to the meta‐analysis. In 1 351 633 people with uncomplicated hypertension using a RAAS inhibitor, calcium channel blocker, or thiazide diuretic in monotherapy, the risk of hospitalization (angiotensin‐converting enzyme inhibitor: HR, 0.76; 95% CI, 0.66–0.87; P<0.001; angiotensin II type‐I receptor blockers: HR, 0.86; 95% CI, 0.77–0.97; P=0.015) and intubation or death (angiotensin‐converting enzyme inhibitor: HR, 0.64; 95% CI, 0.48–0.85; P=0.002; angiotensin II type‐I receptor blockers: HR, 0.74; 95% CI, 0.58–0.95; P=0.019) with COVID‐19 was lower in those using a RAAS inhibitor. However, these protective effects are probably not clinically relevant.
Conclusions
This study reveals the critical risk of bias that exists across almost an entire body of COVID‐19 research, raising an important question: Were research methods and/or peer‐review processes temporarily weakened during the surge of COVID‐19 research or is this lack of rigor a systemic problem that also exists outside pandemic‐based research?
Registration
URL: www.crd.york.ac.uk/prospero/; Unique identifier: CRD42021237859.
Keywords: angiotensin receptor blockers, angiotensin‐converting enzyme inhibitors, calcium channel blockers, COVID‐19, renin‐aldosterone angiotensin system inhibitors, thiazide diuretics
Subject Categories: Epidemiology, ACE/Angiotension Receptors/Renin Angiotensin System, Hypertension, Mortality/Survival, Pharmacology
Nonstandard Abbreviations and Acronyms
- RAAS
renin‐angiotensin aldosterone system
- ROBINS‐I
Risk of Bias in Non‐Randomized Studies—of Interventions
- TZD
thiazide diuretic
Clinical Perspective
What Is New?
This review identified critical biases in 164 of the 169 studies that, until October 21, 2021, evaluated the associations between renin‐angiotensin aldosterone system (RAAS) inhibitor use and COVID‐19 outcomes, biases that could have distorted the evidence upon which health policy was based.
As of December 2021, at least 52 meta‐analyses were conducted against guidelines, pooling studies with critical risk of bias.
Synthesizing two nationwide studies, which used methods that, as best as possible, isolated RAAS inhibitor—COVID‐19 interactions, this meta‐analysis provided further evidence that RAAS inhibitor use does not increase the risk of severe COVID‐19 outcomes.
What Are the Clinical Implications?
The findings of this study support directives from health authorities, early in the pandemic, that users of RAAS inhibitors could continue their treatment safely.
Although bias‐minimizing methods needed to be applied to isolate the interaction between RAAS inhibitor use and COVID‐19, that methodological approach limits the generalizability of these findings; and, thus, it is not known if these findings are applicable to those with underlying comorbidities and those in combination therapy; the proportion of the population who are most at risk of severe COVID‐19 outcomes and who represent the majority of people using RAAS inhibitors.
Since it was found that SARS‐CoV‐2 gains entry into human cells via angiotensin‐converting enzyme (ACE) 2, 1 there has been an inundation of research evaluating if the use of renin‐angiotensin aldosterone system (RAAS) inhibitors increases the risk of a SARS‐CoV‐2 infection and/or severe COVID‐19 outcomes. Those studies, predominantly observational in design, were driven by the notion that RAAS inhibitors, such as ACE inhibitors and or angiotensin II type‐I receptor blockers (ARBs), may upregulate the expression of ACE2. 2 , 3 , 4 As the pandemic progressed, however, it became evident that, like a lot of COVID‐19 research, many RAAS inhibitor—COVID‐19 studies have suffered from the speed at which they were conducted 5 ; often using nonrepresentative samples where the risk of selection (ie, collider) bias is increased. 6 At least 52 meta‐analyses have assessed associations between RAAS inhibitor use and COVID‐19 risk by collating parts of that body of research. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 However, little attention has been paid to selection bias, or other biases for that matter, which may distort the interaction between RAAS inhibitor use and COVID‐19.
Given the sheer volume of previous research, it is understandable if the appeal of additional RAAS inhibitor—COVID‐19 studies may be subsiding. However, in the interest of improving the methods used in observational research and, subsequently, enhancing its value, there is an urgent need to review how the scientific community has attempted to address this issue; to what extent does bias exist across that body of RAAS inhibitor—COVID‐19 research and does that bias significantly distort the interaction between RAAS inhibitor use and COVID‐19 risk? Accordingly, this study retested the hypothesis that RAAS inhibitor use is associated with important, severe COVID‐19 outcomes, using only data from observational studies without critical risk of bias.
METHODS
The data that support the findings of this study are available within the article. The protocol of this systematic review and meta‐analysis was registered on PROSPERO (Registration number: CRD42021237859) before the study commenced and is available in full on the National Institute for Health Research International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/PROSPERO). This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist (Table S1).
Data Sources and Searches
Systematic searches were performed in MEDLINE, EMBASE, and CINAHL databases from December 1, 2019, near to when the first case of SARS‐CoV‐2 was identified, until October 21, 2021. A combination of subject headings for COVID‐19 and RAAS inhibitors was used (Table S2). Searches were limited to “human” studies only. A manual search of the citations included in identified reviews and articles selected for full‐text retrieval was also performed.
Study Selection, Inclusion and Exclusion Criteria
Two investigators (J. L. and F. C. T.) independently performed study selection using Covidence, an online, Cochrane approved, software designed for conducting systematic reviews. 59 Discrepancies in inclusion or exclusion were solved through consultation with a third investigator (J. S.). To address the risk of bias in all observational studies of RAAS inhibitor—COVID‐19 associations, the systematic review initially identified studies that compared the risk of infection with COVID‐19 and/or severe COVID‐19 outcomes (eg, hospitalization, admission to an intensive care unit, intubation and/or death with COVID‐19) between those using an ACE inhibitor or an ARB and those not using a RAAS inhibitor. Only data from studies on people aged ≥18 years were included. Review articles were excluded, but as stated previously, their reference lists were screened. Studies indexed to preprint servers only, which are not certified by peer review, were excluded. Conference abstracts were also excluded. Finally, inclusion was limited to studies originally published in the English language or where translated copies had been made available.
Risk of Bias Assessment
Given that this review focussed on observational studies, the risk of bias assessment for each study was performed independently by J. L. and F. C. T. using the Risk of Bias in Non‐Randomized Studies—of Interventions (ROBINS‐I), developed by the Cochrane Bias Methods Group and the Cochrane Non‐Randomized Studies Methods Group. 60 The ROBINS‐I tool includes seven domains of potential bias: (1) bias due to confounding, (2) bias in selection of participants into the study, (3) bias in the classification of interventions, (4) bias due to deviations from intended interventions, (5) bias due to missing data, (6) bias in the measurements of outcomes, and (7) bias in selection of the reported result. 60
A study was deemed to have a low risk of bias overall if it was judged to have a low risk of bias across all domains; a moderate risk of bias overall if it was judged to have a low or moderate risk of bias across all domains; a serious risk of bias overall if it was judged to have a serious risk of bias in at least one domain, but not at a critical risk of bias in any other domain; and a critical risk of bias overall if it was judged to have a critical risk of bias in at least one domain. 60 If there was nothing indicating a serious or critical risk of bias, but there was a lack of information to make a judgment in any of the domains, a study was deemed to have “no information” regarding its risk of bias. 60 Discrepancies in bias classification were resolved by discussion. As recommended, any study with a critical risk of bias was excluded from the subsequent synthesis and analysis. 60
Outcomes
Although risk of bias was assessed in studies of the association between RAAS inhibitor use and the risk of infection, it was planned from the outset that only severe COVID‐19 outcomes (eg, hospitalization, admission to an intensive care unit, intubation, death) would be evaluated by the meta‐analysis. Indeed, severe outcomes are most relevant considering that they are the burden to health care systems, whereas risk of infection data have an unknown potential for bias because of changes in COVID‐19 testing strategies in outpatient care and, likely, a high rate of missed cases. Severe outcomes that could be pooled from the eligible studies included hospitalization and a combination of intubation and death with COVID‐19.
Data Extraction
The main characteristics of each study eligible for inclusion in the meta‐analysis were summarized in duplicate by J. L. and F. C. T. into a preformatted spreadsheet. These characteristics included (1) first author names and year of publication, (2) country, (3) study design, (4) sample size, (5) comorbidities, (6) number of relevant severe COVID‐19 events, and (7) the weighted estimates for each outcome of interest.
Statistical Analysis
The weighted hazard ratios (HR) and the corresponding 95% CI detailing the association between using an ACE inhibitor or an ARB in monotherapy and hospitalization, intubation, or death with COVID‐19 were pooled in fixed‐effects meta‐analyses. Those using a RAAS inhibitor in monotherapy were compared with those using a non‐RAAS inhibitor in monotherapy (ie, a calcium channel blocker or thiazide diuretic [TZD]). A fixed‐effects meta‐analysis was chosen as the primary analysis given the low number of studies included in the meta‐analyses. Indeed, a random‐effects meta‐analysis may not adequately estimate the heterogeneity and weights when so few studies are available. 61 Recognizing that there are contrasting opinions as to when it is appropriate to use either a fixed‐ or random‐effects model, a random‐effects meta‐analysis was conducted as a secondary (sensitivity) analysis. Heterogeneity was quantified using the I² statistic (I²>50%) and tested using Cochran’s Q statistic (P<0.10). 62 Publication bias was not assessed as there were fewer than 10 studies available for the analysis. 63 A P value of <0.05 was considered statistically significant. Analyses were performed using R, version 4.0.0 (R‐Core Team, Vienna, Austria). 64
Certainty of the Evidence Assessment
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) was performed in duplicate by J. L. and F. C. T. to provide an assessment of the quality of (ie, certainty of) the evidence produced by the meta‐analyses. The GRADE’s official software package, GRADEpro Guideline Development Tool (McMaster University and Evidence Prime Inc.), 65 was used to summarize the findings of the meta‐analyses and rate the certainty of the evidence for each outcome as high, moderate, low, or very low. 60 More information about the GRADE can be found in Data S1.
RESULTS
Study Selection and Risk of Bias Analysis
The systematic search identified 169 observational studies that assessed the associations between RAAS inhibitor use and the risk of contracting a SARS‐CoV‐2 infection and/or experiencing severe COVID‐19 outcomes (Figure 1). 8 , 19 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 Of those 169 studies, 164 had critical risks of bias and were excluded from the meta‐analysis (Figure 2). Among these 164 studies, critical biases were most commonly due to confounding bias (n=157), selection bias (n=146), and bias due to deviations from the intended interventions (n=67).
Figure 1. Flow diagram of the study selection process.
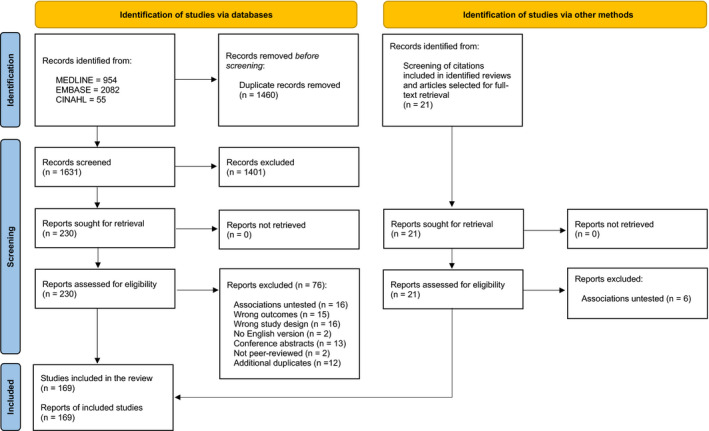
Figure 2. Risk of bias assessment using the Risk of Bias in Non‐Randomized Studies—of Interventions (ROBINS‐I).
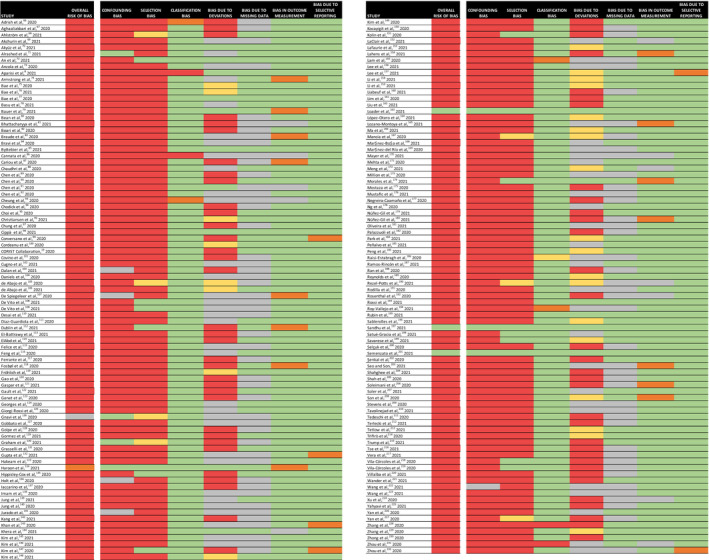
The risk of bias is indicated as an overall risk of bias (ie, across all domains), as well as for each separate domain of potential bias, in each study that met the inclusion criteria of this systematic review and meta‐analysis. In total, 164 out of 169 were judged to have a critical risk of bias and were excluded from the meta‐analysis. Green indicates a low risk of bias, yellow indicates a moderate risk of bias, orange indicates a serious risk of bias, red indicates a critical risk of bias, and grey indicates that there was a lack of information in the study to make a judgment on the risk of bias for that specific domain.
The risk of bias in the classification of the interventions, the measurement of the outcomes, and the selection of the reported result was predominantly low across the entire body of research. Notably, a substantial number of studies failed to provide information about how missing data were handled (n=90) or if important cointerventions (ie, other antihypertensive therapies) were addressed (n=60).
Of the five studies without critical risks of bias, two studies assessed only the association between RAAS inhibitor use and risk of infection (a nonsevere outcome that does not burden health care systems) and, as planned, were not included in the subsequent data synthesis. 126 , 134 A third study without critical bias had eligible data in its secondary analysis, but the event rates for the relevant outcomes were not available 197 ; and, thus, it could not be pooled in the meta‐analysis.
Study Characteristics
Two nationwide studies, one each from France and Sweden, could be included in the meta‐analysis (Table 1). 163 , 201 Although the study from France also completed analyses on those using a combination of antihypertensive therapies, the meta‐analysis considered only outcomes commonly assessed in each study and, thus, included data from all French (n=1 186 987) and Swedish (n=164 655) residents with uncomplicated hypertension who used either an ACE inhibitor, ARB, calcium channel blocker, or TZD in monotherapy (Note: data for French citizens using a TZD in monotherapy were not available). Given that as‐treated data were available only in the study from Sweden, only intention‐to‐treat data were used in the meta‐analysis. Both studies excluded those with known cardiovascular and/or kidney diseases in order to limit confounding bias. The follow‐up period was similar in both the French and Swedish studies, running from February 15, 2020, to June 7, 2020, and from January 1, 2020, to June 23, 2020, respectively; covering the first wave of the COVID‐19 pandemic in each country.
Table 1.
Main Characteristics of the Studies Included in the Meta‐Analysis
| Study | Country | Study period | Population* | Comorbidities | Hospitalization with COVID‐19 | Intubation or death with COVID‐19 † |
|---|---|---|---|---|---|---|
| Loader et al, 163 2021 | Sweden | January 1, 2020–June 23, 2020 |
All residents in Sweden (n=164 655) with uncomplicated hypertension using, in monotherapy, an ACE inhibitor (n=47 998) ARB (n=68 239) CCB or TZD (n=48 418) |
Those with preexisting cardiovascular disease and kidney diseases were excluded |
ACE inhibitor (n=94) ARB (n=135) CCB or TZD (n=107) |
ACE inhibitor (n=16) ARB (n=19) CCB or TZD (n=26) |
| Semenzato et al, 201 2021 | France | February 15, 2020–June 7, 2020 |
All residents in France (n=1 186 987) with uncomplicated hypertension using, in monotherapy, an ACE inhibitor (n=353 236) ARB (n=582 031) CCB (n=251 720) |
Those with diabetes, cardiovascular disease, chronic respiratory disease, and/or chronic renal failure in the 5 years before the study were excluded. |
ACE inhibitor (n=340) ARB (n=690) CCB (n=384) |
ACE inhibitor (n=72) ARB (n=148) CCB (n=99) |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II type‐I receptor blocker; CCB, calcium channel blocker; and TZD, thiazide diuretic.
Although only data for treatment by monotherapy are presented in the table, it should be noted that Semenzato et al. (2021) also conducted analyses on those in combination therapy.
Intubation was only an outcome in the study by Semenzato et al. (2021), meaning only deaths were recorded in Loader et al. (2021).
Meta‐Analyses
The fixed‐effects meta‐analyses indicate that in those using an ACE inhibitor or an ARB in monotherapy, there is a lower risk of hospitalization (ACE inhibitor: HR, 0.76; 95% CI, 0.66–0.87; P<0.001; ARB: HR, 0.86; 95% CI, 0.77–0.97; P=0.015) and intubation or death (ACE inhibitor: HR, 0.64; 95% CI, 0.48–0.85; P=0.002; ARB: HR, 0.74; 95% CI, 0.58–0.95; P=0.019) with COVID‐19 (Figure 3), when compared with those using a calcium channel blocker or TZD in monotherapy. The heterogeneity in each mortality analysis was most likely driven by the low number of events in the study from Sweden. The direction of the estimates varied little when a random‐effects meta‐analysis was used (Figure S1).
Figure 3. Forest plots for each outcome assessed in the fixed‐effects meta‐analyses.
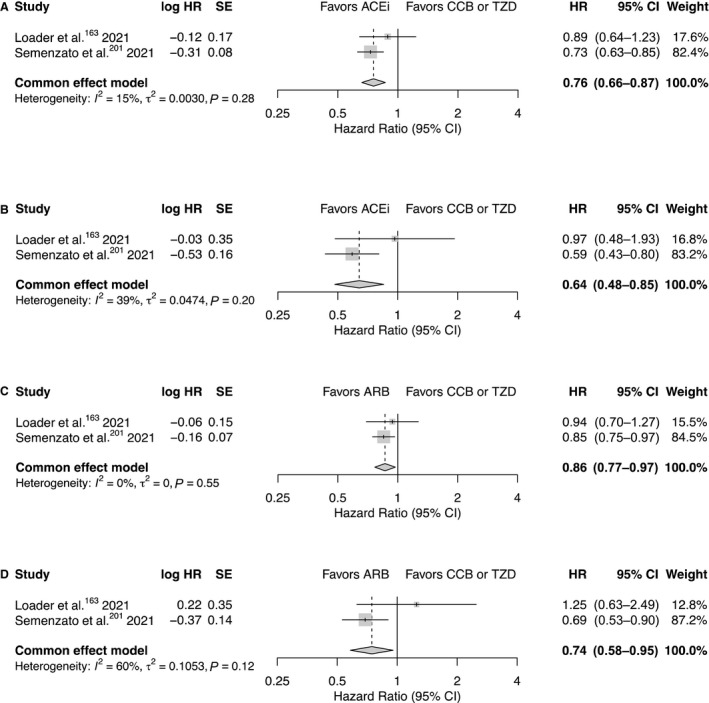
Presented are the associations between (A) the use of an ACE inhibitor in monotherapy and hospitalization, (B) the use of an ACE inhibitor in monotherapy and intubation or death, (C) the use of an ARB in monotherapy and hospitalization and (D) the use of an ARB in monotherapy and intubation or death. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II type‐I receptor blocker; CCB, calcium channel blocker; HR, hazard ratio; and TZD, thiazide diuretic.
Certainty of the Evidence
The GRADE indicated that the protective effects detected by the meta‐analyses are probably not clinically relevant, that the risk of hospitalization, intubation, or death varies little between those using a RAAS inhibitor and those using a calcium channel blocker or TZD (Table 2). Although the meta‐analyses were based on observational studies, certainty in the evidence for each outcome began with a high rating because of the use of the ROBINS‐I. 60 There were no reasons to downgrade the certainty of the evidence for each of the hospitalization outcomes. However, given that only one of two studies provided intubation data in the intubation or death outcomes, certainty of the evidence for these outcomes was downgraded by one level to a moderate rating owing to serious indirectness in the outcomes between studies.
Table 2.
Summary of Findings Including the Certainty of the Evidence
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with a CCB or TZD | Risk with an ACE inhibitor or ARB | |||||
| ACE inhibitor use and the risk of hospitalization with COVID‐19 | 164 per 100 000 |
124 per 100 000 (108–142) |
HR 0.76 (0.66–0.87) |
701 372 (2 observational studies) |
⨁⨁⨁⨁ HIGH |
The risk of hospitalization with COVID‐19 differs little between those using an ACE inhibitor and those using a CCB or TZD in monotherapy |
| ACE inhibitor use and the risk of intubation or death with COVID‐19 | 42 per 100 000 |
27 per 100 000 (20–35) |
HR 0.64 (0.48–0.85) |
701 372 (2 observational studies) |
⨁⨁⨁◯ MODERATE†,‡,§ |
The risk of intubation or death with COVID‐19 differs little between those using an ACE inhibitor and those using a CCB or TZD in monotherapy |
| ARB use and the risk of hospitalization with COVID‐19 | 164 per 100 000 |
141 per 100 000 (126–159) |
HR 0.86 (0.77–0.97) |
950 408 (2 observational studies) |
⨁⨁⨁⨁ HIGH |
The risk of hospitalization with COVID‐19 differs little between those using an ARB and those using a CCB or TZD in monotherapy |
| ARB use and the risk of intubation or death with COVID‐19 | 42 per 100 000 |
31 per 100 000 (24–40) |
HR 0.74 (0.58–0.95) |
950 408 (2 observational studies) |
⨁⨁⨁◯ MODERATE†,‡,§ |
The risk of intubation or death with COVID‐19 differs little between those using an ARB and those using a CCB or TZD in monotherapy |
GRADE Working Group grades of evidence—High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II type‐I receptor blocker; CCB, calcium channel blocker; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HR, hazard ratio; and TZD, thiazide diuretic.
*The risk in users of an ACE inhibitor or an ARB (and its 95% CI) is based on the assumed risk in the users of a CCB or TZD and therelative effect of the intervention (and its 95% CI).
Explanations
†A composite outcome of intubation and death was used in the meta‐analysis, but intubation data were not available in Loader et al. (2021), resulting in the certainty of the evidence being downgraded by one level due to serious indirectness.
‡Point estimates vary greatly between each study, but the importance of this is questionable when considering the weighting of each study and does not decrease certainty in the evidence.
§Heterogeneity is most likely explained by the low number of deaths in Loader et al. (2021).
DISCUSSION
This study reveals that current policy regarding the safety of using RAAS inhibitors during the ongoing COVID‐19 pandemic is based almost entirely on a body of research that has severe limitations due to critical risk of bias. However, when only studies without critical risk of bias were pooled, results were consistent with the majority of that body of research; indicating that RAAS inhibitor use does not increase the risk of severe COVID‐19 outcomes.
Critical risk of bias was most often attributed to confounding bias and/or selection bias, found in 164 of 169 observational studies relevant to this meta‐analysis. Confounding bias was introduced mainly by the inclusion of people with preexisting cardiovascular and kidney diseases, confounding factors that are inherently uncontrollable when aiming to isolate the effect of RAAS inhibitor use on COVID‐19 outcomes. Selection bias was introduced by the inclusion of samples restricted to people who were tested for/tested positive to a SARS‐CoV‐2 infection and/or who had been hospitalized due to COVID‐19; sampling strategies that form cohorts unrepresentative of the general population.
These biases have the potential to create a spurious within‐sample association between two variables, so called collider bias (eg, in the context of RAAS inhibitor—COVID‐19 studies: frailty due to cardiovascular disease, with a high likelihood of being prescribed a RAAS inhibitor; and frailty due to an adverse COVID‐19 course), that affects the probability of being included in the sample (eg, being hospitalized). 6 To minimize such biases in the context of RAAS inhibitor—COVID‐19 observational research, a primary prevention sample of yet uninfected people using RAAS inhibitors or a relevant comparator drug class needs to be studied in order to isolate the interaction between RAAS inhibitor use and COVID‐19, not in those already affected by the virus. 6
Ultimately, only five observational studies used bias‐minimized study designs, two of which could be included in the meta‐analysis. The conclusion from this research, that RAAS inhibitor use does not increase the risk of severe COVID‐19 outcomes, is consistent with the eight randomized controlled trials that have evaluated RAAS inhibitor—COVID‐19 associations thus far. 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 Although the 164 studies with critical biases cannot isolate the interaction between RAAS inhibitor use and COVID‐19, the value of that research should not be discounted entirely. Indeed, those studies combined contributed to a detailed characterization of the population who experienced a severe COVID‐19 disease course, identifying the people (eg, elderly people, people with comorbidities) who need more attention or greater protection (eg, prioritized vaccination) during the ongoing pandemic.
The exclusion of people with preexisting cardiovascular and kidney diseases from the studies pooled in the meta‐analyses is likely to prompt questions about the limitations of this study: Do these findings apply to those with such comorbidities? Would these findings not be more relevant if the analyses include those with comorbidities, those who are more at risk of severe a COVID‐19 disease course? Indeed, it is unknown whether the findings of this meta‐analysis extend to those with underlying comorbidities, the proportion of the population who are most at risk of severe COVID‐19 outcomes and who represent the majority of people using RAAS inhibitors. Although it is important that this limitation is acknowledged, the fact remains that people with preexisting cardiovascular and kidney diseases could introduce confounding so intractable that statistical methods will not be able to control for this bias, 241 necessitating the exclusion of those people to isolate any interaction between RAAS inhibitors and COVID‐19. This methodological consideration represents an effort to produce observational research that, as best as possible, emulates a randomized controlled trial, a methodological principle that seems to have been overlooked in many drug‐safety and effectiveness studies during the COVID‐19 pandemic, 241 not only those pertaining to RAAS inhibitors.
Other potential limitations must also be addressed. This meta‐analysis did not conduct an analysis including people on combination therapy with antihypertensive drugs. However, given the results from the nationwide study in France, 201 it would appear that these findings based on monotherapy data could be extended to those on combination therapy. Owing to data availability, only intention‐to‐treat data were used in the analysis and variations in the prescribed RAAS inhibitor dose were not addressed by the meta‐analysis. Thus, it is unclear how an as‐treated analysis would affect the findings. However, the nationwide study from Sweden indicated that there is not much variation in the estimates between an intention‐to‐treat and an as‐treated analysis. 163 Finally, both studies in the meta‐analysis (like most RAAS inhibitor—COVID‐19 research) covered only the first wave of the pandemic, where event rates were relatively low compared with subsequent waves, and when early variants of the virus existed, possibly limiting the data in terms of their relevance to the evolving COVID‐19 situation.
This study was not the first systematic review and meta‐analysis to assess the associations between RAAS inhibitor use and COVID‐19 outcomes. As of December 2021, at least 52 meta‐analyses had been conducted, 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 each of which pooled studies that have a critical risk of bias. Critical biases limit confidence in the evidence produced by these meta‐analyses as much as they do in each of the studies that they were based on. Current guidelines from the Cochrane Collaboration instruct that these meta‐analyses should not have been conducted, that studies with critical risk of bias should not be synthesized. 242 However, only six of the 52 previous meta‐analyses followed current guidelines and used the ROBINS‐I to assess risk of bias. 16 , 21 , 28 , 35 , 36 , 39 It is unclear as to why the authors of five of those six studies did not identify any critical risks of bias and proceeded with their meta‐analyses. One meta‐analysis, however, deviated from the guidelines, establishing their own unstandardized scoring system for the ROBINS‐I. 21
Of the other 46 meta‐analyses, only 18 referred to having completed a risk of bias assessment. Whether they recognized it or not, 34 studies used the Newcastle‐Ottawa Scale for assessing the risk of bias. The Newcastle‐Ottawa Scale, which has not been recommended by the Cochrane Collaboration since well before the COVID‐19 pandemic (in a now archived version of the guidelines), provides a quality score where a higher value is interpreted as a lower risk of bias. Although this scale might detect a critical risk of bias in one domain, a study may still be deemed to be of high quality overall and, thus, have a low risk of bias overall because of strengths in other domains; essentially allowing for an important, potentially association‐distorting bias(es) to go overlooked and explaining why some studies proceeded with their meta‐analyses. In contrast, the ROBINS‐I recognises that a critical risk of bias needs to exist in only one domain to limit confidence in an entire study and to provide reason for it to be excluded from a data synthesis. The Cochrane Collaboration’s transition from the Newcastle‐Ottawa Scale to the ROBINS‐I further reflects the need for more rigor in the design of observational research.
Three meta‐analyses acknowledged use of a risk of bias assessment tool that is integrated into the GRADE. This tool, by admission of its creators, was not as comprehensive as other methods available at the time (Note: created long before the development for the ROBINS‐I) 243 ; and like the Newcastle‐Ottawa Scale, it also overlooks important sources of bias. Only eight out of 52 meta‐analyses used the GRADE to summarize their findings and determine the certainty of their evidence; a process that is crucial in being able to properly interpret the clinical importance of findings from a meta‐analysis (eg, as demonstrated in this study: the difference between concluding a protective effect and no effect). Collectively, this poor methodology demonstrates a lack of knowledge around the current guidelines for systematic reviews and meta‐analyses, among authors, reviewers, and journal editors alike.
CONCLUSIONS
Despite wavering interest, additional studies related to COVID‐19 risk are inevitable, emphasizing the urgent need for this present systematic review and meta‐analysis. Indeed, to improve future research, the inadequate methodologies used by almost an entire body of observational research, as well as by the meta‐analyses that collated those studies, needed to be highlighted. If further RAAS—COVID‐19 observational studies are to be conducted, it would be interesting if they extended follow‐up to include subsequent waves of the pandemic, where event rates were substantially higher and when new variants of COVID‐19 exist(ed); evaluating if associations change over time with variations in infection rates and in the virus. That research must address the methodological issues (eg, confounding bias and selection bias) identified in most previous studies in order to, as best as possible, isolate the interaction between RAAS inhibitor use and COVID‐19. Researchers should also recognize that the methodological principles needed to improve RAAS—COVID‐19 studies extend to other drug safety and effectiveness studies; methodological approaches that are crucial to enhancing the scientific value of observational research.
In summary, this study presents data that, fortunately, support directives from health authorities early in the pandemic, that antihypertensive therapies could be continued safely as per normal. However, it is not certain whether the effect estimates would remain consistent throughout subsequent waves of the pandemic or whether the findings are applicable to those with underlying comorbidities. Most significantly, this study reveals the extent of bias in RAAS inhibitor—COVID‐19 studies, showing how wrong things can go when there is carelessness in research; that poorly designed studies continue, with little question, to direct subsequent research and health policies alike. This raises an important question: Have research methods and/or peer review processes been temporarily weakened during the surge of COVID‐19 research, potentially because of a desire to be the first published or to be the first to publish, or is this lack of rigor a systemic problem that also exists outside pandemic‐based research?
Sources of Funding
This project (Dr Jordan Loader) has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska‐Curie grant agreement no. 898829. This research was also supported by funding from the Swedish Heart‐Lung Foundation and Anders Wiklöf.
Disclosures
Dr Jordan Loader has completed medical writing services, separate of this study, for AstraZeneca; Professor Johan Sundström reports stock ownership in companies providing services to Itrim, Amgen, Janssen, Novo Nordisk, Eli Lilly, Boehringer, Bayer, Pfizer, Takeda, and AstraZeneca. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S2
Figure S1
Acknowledgments
Author contributions: Dr Jordan Loader developed the concept and the design of the study. Dr Loader and Miss Frances Taylor were responsible for acquisition of the data. Dr Loader and Dr Erik Lampa were responsible for data analysis. All authors, in addition to Professor Johan Sundström, contributed to the interpretation of the data and to the production of the article. All authors approved the final version of the article.
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N‐H, Nitsche A, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461 [DOI] [PubMed] [Google Scholar]
- 4. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin‐converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a [DOI] [PubMed] [Google Scholar]
- 5. Bramstedt KA. The carnage of substandard research during the COVID‐19 pandemic: a call for quality. J Med Ethics. 2020;46:803–807. doi: 10.1136/medethics-2020-106494 [DOI] [PubMed] [Google Scholar]
- 6. Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L, Sharp GC, Sterne J, Palmer TM, Davey Smith G, et al. Collider bias undermines our understanding of COVID‐19 disease risk and severity. Nat Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alamer AA, Almulhim AS, Alrashed AA, Abraham I. Mortality, severity, and hospital admission among COVID‐19 patients with ACEI/ARB use: a meta‐analysis stratifying countries based on response to the first wave of the pandemic. Healthcare (Basel). 2021;9:127. doi: 10.3390/healthcare9020127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aparisi Á, Catalá P, Amat‐Santos IJ, Marcos‐Mangas M, López‐Otero D, Veras C, López‐Pais J, Cabezón‐Villalba G, Cacho Antonio CE, Candela J, et al. Chronic use of renin–angiotensin–aldosterone inhibitors in hypertensive COVID‐19 patients: results from a Spanish registry and meta‐analysis. Med Clin (Barc). 2021;158:315–323. doi: 10.1016/j.medcli.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baral R, Tsampasian V, Debski M, Moran B, Garg P, Clark A, Vassiliou VS. Association between renin‐angiotensin‐aldosterone system inhibitors and clinical outcomes in patients with COVID‐19: a systematic review and meta‐analysis. JAMA Netw Open. 2021;4:e213594. doi: 10.1001/jamanetworkopen.2021.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baral R, White M, Vassiliou VS. Effect of renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19: a systematic review and meta‐analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22:61. doi: 10.1007/s11883-020-00880-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barochiner J, Martínez R. Use of inhibitors of the renin‐angiotensin system in hypertensive patients and COVID‐19 severity: a systematic review and meta‐analysis. J Clin Pharm Ther. 2020;45:1244–1252. doi: 10.1111/jcpt.13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bavishi C, Whelton PK, Mancia G, Corrao G, Messerli FH. Renin‐angiotensin‐system inhibitors and all‐cause mortality in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. J Hypertens. 2021;39:784–794. doi: 10.1097/HJH.0000000000002784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bezabih YM, Bezabih A, Alamneh E, Peterson GM, Bezabhe W. Comparison of renin–angiotensin–aldosterone system inhibitors with other antihypertensives in association with coronavirus disease‐19 clinical outcomes. BMC Infect Dis. 2021;21:527. doi: 10.1186/s12879-021-06088-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biswas M, Kali MSK. Association of angiotensin‐converting enzyme inhibitors and angiotensin‐receptor blockers with risk of mortality, severity or SARS‐CoV‐2 test positivity in COVID‐19 patients: meta‐analysis. Sci Rep. 2021;11:5012. doi: 10.1038/s41598-021-84678-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai XJ, Tay JCK, Kui SL, Tin AS, Tan VH. Impact of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on in‐hospital mortality among patients with COVID‐19: a systematic review and meta‐analysis. Singapore Med J. 2021;62:563–567. doi: 10.11622/smedj.2020159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caldeira D, Alves M, Gouveia e Melo R, Silvério António P, Cunha N, Nunes‐Ferreira A, Prada L, Costa J, Pinto FJ. Angiotensin‐converting enzyme inhibitors and angiotensin‐receptor blockers and the risk of COVID‐19 infection or severe disease: systematic review and meta‐analysis. Int J Cardiol Heart Vasc. 2020;31:100627. doi: 10.1016/j.ijcha.2020.100627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan C‐K, Huang Y‐S, Liao H‐W, Tsai I‐J, Sun C‐Y, Pan H‐C, Chueh JS, Wang J‐T, Wu V‐C, Chu T‐S, et al. Renin‐angiotensin‐aldosterone system inhibitors and risks of severe acute respiratory syndrome coronavirus 2 infection: a systematic review and meta‐analysis. Hypertension. 2020;76:1563–1571. doi: 10.1161/HYPERTENSIONAHA.120.15989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu C, Zeng S, Hasan AA, Hocher C‐F, Krämer BK, Hocher B. Comparison of infection risks and clinical outcomes in patients with and without SARS‐CoV‐2 lung infection under renin‐angiotensin‐aldosterone system blockade: systematic review and meta‐analysis. Br J Clin Pharmacol. 2021;87:2475–2492. doi: 10.1111/bcp.14660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. COVID‐19 RISk and Treatments (CORIST) Collaboration . RAAS inhibitors are not associated with mortality in COVID‐19 patients: findings from an observational multicenter study in Italy and a meta‐analysis of 19 studies. Vascul Pharmacol. 2020;135:106805. doi: 10.1016/j.vph.2020.106805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dai X‐C, An Z‐Y, Wang Z‐Y, Wang Z‐Z, Wang Y‐R. Associations between the use of renin‐angiotensin system inhibitors and the risks of severe COVID‐19 and mortality in COVID‐19 patients with hypertension: a meta‐analysis of observational studies. Front Cardiovasc Med. 2021;8:609857. doi: 10.3389/fcvm.2021.609857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernando ME, Drovandi A, Golledge J. Meta‐analysis of the association between angiotensin pathway inhibitors and COVID‐19 severity and mortality. Syst Rev. 2021;10:243. doi: 10.1186/s13643-021-01802-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flacco ME, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A, Manfredini R, Mantovani LG, Manzoli L. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID‐19: a meta‐analysis. Heart. 2020;106:1519–1524. doi: 10.1136/heartjnl-2020-317336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greco A, Buccheri S, D’Arrigo P, Calderone D, Agnello F, Monte M, Milluzzo RP, Franchina AG, Ingala S, Capodanno D. Outcomes of renin‐angiotensin‐aldosterone system blockers in patients with COVID‐19: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Pharmacother. 2020;6:335–337. doi: 10.1093/ehjcvp/pvaa074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grover A, Oberoi M. A systematic review and meta‐analysis to evaluate the clinical outcomes in COVID‐19 patients on angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2021;7:148–157. doi: 10.1093/ehjcvp/pvaa064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo X, Zhu Y, Hong Y. Decreased mortality of COVID‐19 with renin‐angiotensin‐aldosterone system inhibitors therapy in patients with hypertension: a meta‐analysis. Hypertension. 2020;76:e13–e14. doi: 10.1161/HYPERTENSIONAHA.120.15572 [DOI] [PubMed] [Google Scholar]
- 26. Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID‐19 patients receiving renin‐angiotensin system inhibitors: a systematic review and meta‐analysis. Am J Cardiovasc Drugs. 2020;20:571–590. doi: 10.1007/s40256-020-00439-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hassib M, Hamilton S, Elkhouly A, Li Y, Kaplan AC. Renin‐angiotensin‐aldosterone system inhibitors and COVID‐19: a meta‐analysis and systematic review. Cureus. 2021;13:e13124. doi: 10.7759/cureus.13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jia N, Zhang G, Sun X, Wang Y, Zhao S, Chi W, Dong S, Xia J, Zeng P, Liu D. Influence of angiotensin converting enzyme inhibitors/angiotensin receptor blockers on the risk of all‐cause mortality and other clinical outcomes in patients with confirmed COVID‐19: a systemic review and meta‐analysis. J Clin Hypertens (Greenwich). 2021;23:1651–1663. doi: 10.1111/jch.14329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kashour T, Bin Abdulhak AA, Tlayjeh H, Hassett LC, Noman A, Mohsen A, Al‐Mallah MH, Tleyjeh IM. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers and mortality among COVID‐19 patients: a systematic review and meta‐analysis. Am J Ther. 2020. [epub ahead of print]. doi: 10.1097/MJT.0000000000001281 [DOI] [PubMed] [Google Scholar]
- 30. Kaur U, Chakrabarti SS, Patel TK. Renin‐angiotensin‐aldosterone system blockers and region‐specific variations in COVID‐19 outcomes: findings from a systematic review and meta‐analysis. Ther Adv Drug Saf. 2021;12:20420986211011344. doi: 10.1177/20420986211011345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerneis M, Ferrante A, Guedeney P, Vicaut E, Montalescot G. Severe acute respiratory syndrome coronavirus 2 and renin‐angiotensin system blockers: a review and pooled analysis. Arch Cardiovasc Dis. 2020;113:797–810. doi: 10.1016/j.acvd.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koshy AN, Murphy AC, Farouque O, Ramchand J, Burrell LM, Yudi MB. Renin‐angiotensin system inhibition and risk of infection and mortality in COVID‐19: a systematic review and meta‐analysis. Intern Med J. 2020;50:1468–1474. doi: 10.1111/imj.15002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurdi A, Abutheraa N, Akil L, Godman B. A systematic review and meta‐analysis of the use of renin‐angiotensin system drugs and COVID‐19 clinical outcomes: what is the evidence so far? Pharmacol Res Perspect. 2020;8:e00666. doi: 10.1002/prp2.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laurentius A, Mendel B, Prakoso R. Clinical outcome of renin‐angiotensin‐aldosterone system blockers in treatment of hypertensive patients with COVID‐19: a systematic review and meta‐analysis. Egypt Heart J. 2021;73:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee HW, Yoon C‐H, Jang EJ, Lee C‐H. Renin‐angiotensin system blocker and outcomes of COVID‐19: a systematic review and meta‐analysis. Thorax. 2021;76:479–486. doi: 10.1136/thoraxjnl-2020-215322 [DOI] [PubMed] [Google Scholar]
- 36. Lee MMY, Docherty KF, Sattar N, Mehta N, Kalra A, Nowacki AS, Solomon SD, Vaduganathan M, Petrie MC, Jhund PS, et al. Renin‐angiotensin system blockers, risk of SARS‐CoV‐2 infection and outcomes from CoViD‐19: systematic review and meta‐analysis. Eur Heart J Cardiovasc Pharmacother. 2022;8:165–178. doi: 10.1093/ehjcvp/pvaa138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee T, Cau A, Cheng MP, Levin A, Lee TC, Vinh DC, Lamontagne F, Singer J, Walley KR, Murthy S, et al. Angiotensin receptor blockers and angiotensin‐converting enzyme inhibitors in COVID‐19: meta‐analysis/meta‐regression adjusted for confounding factors. CJC Open. 2021;3:965–975. doi: 10.1016/j.cjco.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu X, Long C, Xiong Q, Chen C, Ma J, Su Y, Hong K. Association of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with risk of COVID‐19, inflammation level, severity, and death in patients with COVID‐19: a rapid systematic review and meta‐analysis. Clin Cardiol. 2020. doi: 10.1002/clc.23421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lo KB, Bhargav R, Salacup G, Pelayo J, Albano J, McCullough PA, Rangaswami J. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers and outcomes in patients with COVID‐19: a systematic review and meta‐analysis. Expert Rev Cardiovasc Ther. 2020;18:919–930. doi: 10.1080/14779072.2020.1826308 [DOI] [PubMed] [Google Scholar]
- 40. Ma Z, Wang M‐P, Liu L, Yu S, Wu T‐R, Zhao L, Zhang Y‐P, Liang H‐F, Yang X‐C. Does taking an angiotensin inhibitor increase the risk for COVID‐19?—a systematic review and meta‐analysis. Aging (Albany NY). 2021;13:10853–10865. doi: 10.18632/aging.202902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mackey K, King VJ, Gurley S, Kiefer M, Liederbauer E, Vela K, Sonnen P, Kansagara D. Risks and impact of angiotensin‐converting enzyme inhibitors or angiotensin‐receptor blockers on SARS‐CoV‐2 infection in adults: a living systematic review. Ann Intern Med. 2020;173:195–203. doi: 10.7326/M20-1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Megaly M, Glogoza M. Renin‐angiotensin system antagonists are associated with lower mortality in hypertensive patients with COVID‐19. Scott Med J. 2020;65:123–126. doi: 10.1177/0036933020949219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nunes JPL. Mortality and use of angiotensin‐converting enzyme inhibitors in COVID 19 disease: a systematic review. Porto Biomed J. 2020;5:e085. doi: 10.1097/j.pbj.0000000000000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patoulias D, Katsimardou A, Stavropoulos K, Imprialos K, Kalogirou M‐S, Doumas M. Renin‐angiotensin system inhibitors and COVID‐19: a systematic review and meta‐analysis. Evidence for significant geographical disparities. Curr Hypertens Rep. 2020;22:90. doi: 10.1007/s11906-020-01101-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pirola CJ, Sookoian S. Estimation of renin‐angiotensin‐aldosterone‐system (RAAS)‐inhibitor effect on COVID‐19 outcome: a meta‐analysis. J Infect. 2020;81:276–281. doi: 10.1016/j.jinf.2020.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pranata R, Permana H, Huang I, Lim MA, Soetedjo NNM, Supriyadi R, Soeroto AY, Alkatiri AA, Firman D, Lukito AA. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Diabetes Metab Syndr. 2020;14:983–990. doi: 10.1016/j.dsx.2020.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ren L, Yu S, Xu W, Overton JL, Chiamvimonvat N, Thai PN. Lack of association of antihypertensive drugs with the risk and severity of COVID‐19: a meta‐analysis. J Cardiol. 2021;77:482–491. doi: 10.1016/j.jjcc.2020.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sattar Y, Mukuntharaj P, Zghouzi M, Suleiman A‐R, Attique H, Ullah W, Sana MK, Zaher N, Mehmood M, Doshi RP, et al. Safety and efficacy of renin–angiotensin–aldosterone system inhibitors in COVID‐19 population. High Blood Press Cardiovasc Prev. 2021;28:405–416. doi: 10.1007/s40292-021-00462-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ssentongo AE, Ssentongo P, Heilbrunn ES, Lekoubou A, Du P, Liao D, Oh JS, Chinchilli VM. Renin‐angiotensin‐aldosterone system inhibitors and the risk of mortality in patients with hypertension hospitalised for COVID‐19: systematic review and meta‐analysis. Open Heart. 2020;7:e001353. doi: 10.1136/openhrt-2020-001353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tleyjeh IM, Bin Abdulhak AA, Tlayjeh H, Al‐Mallah MH, Sohail MR, Hassett LC, Siller‐Matula JM, Kashour T. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers and the risk of SARS‐CoV‐2 infection or hospitalization with COVID‐19 disease: a systematic review and meta‐analysis. Am J Ther. 2020;29:e74–e84. doi: 10.1097/MJT.0000000000001319 [DOI] [PubMed] [Google Scholar]
- 51. Usman MS, Siddiqi TJ, Khan MS, Ahmed A, Ali SS, Michos ED, Hall ME, Krasuski RA, Greene SJ, Butler J, et al. A meta‐analysis of the relationship between renin‐angiotensin‐aldosterone system inhibitors and COVID‐19. Am J Cardiol. 2020;130:159–161. doi: 10.1016/j.amjcard.2020.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Chen B, Li Y, Zhang L, Wang Y, Yang S, Xiao X, Qin Q. The use of renin‐angiotensin‐aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID‐19 patients: a systematic review and meta‐analysis. J Med Virol. 2021;93:1370–1377. doi: 10.1002/jmv.26625 [DOI] [PubMed] [Google Scholar]
- 53. Xie Q, Tang S, Li Y. The divergent protective effects of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on clinical outcomes of coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Ann Palliat Med. 2021:apm‐21‐972. doi: 10.21037/apm-21-972 [DOI] [PubMed] [Google Scholar]
- 54. Xu J, Teng Y, Shang L, Gu X, Fan G, Chen Y, Tian R, Zhang S, Cao B. The effect of prior angiotensin‐converting enzyme inhibitor and angiotensin receptor blocker treatment on coronavirus disease 2019 (COVID‐19) susceptibility and outcome: a systematic review and meta‐analysis. Clin Infect Dis. 2021;72:e901–e913. doi: 10.1093/cid/ciaa1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xue Y, Sun S, Cai J, Zeng L, Wang S, Wang S, Li J, Sun L, Huo J. Effects of ACEI and ARB on COVID‐19 patients: a meta‐analysis. J Renin Angiotensin Aldosterone Syst. 2020;21:1470320320981321. doi: 10.1177/1470320320981321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yokoyama Y, Aikawa T, Takagi H, Briasoulis A, Kuno T. Association of renin‐angiotensin‐aldosterone system inhibitors with mortality and testing positive of COVID‐19: meta‐analysis. J Med Virol. 2021;93:2084–2089. doi: 10.1002/jmv.26588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang G, Wu Y, Xu R, Du X. Effects of renin‐angiotensin‐aldosterone system inhibitors on disease severity and mortality in patients with COVID‐19: a meta‐analysis. J Med Virol. 2021;93:2287–2300. doi: 10.1002/jmv.26695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang X, Yu J, Pan L‐Y, Jiang H‐Y. ACEI/ARB use and risk of infection or severity or mortality of COVID‐19: a systematic review and meta‐analysis. Pharmacol Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Covidence . Available at: https://www.covidence.org/. Accessed June 29, 2017
- 60. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available at: www.training.cochrane.org/handbook. Accessed June 18, 2021.
- 61. Nikolakopoulou A, Mavridis D, Salanti G. Demystifying fixed and random effects meta‐analysis. Evid Based Ment Health. 2014;17:53–57. doi: 10.1136/eb-2014-101795 [DOI] [PubMed] [Google Scholar]
- 62. Loader J, Khouri C, Taylor F, Stewart S, Lorenzen C, Cracowski J‐L, Walther G, Roustit M. The continuums of impairment in vascular reactivity across the spectrum of cardiometabolic health: a systematic review and network meta‐analysis. Obes Rev. 2019;20:906–920. doi: 10.1111/obr.12831 [DOI] [PubMed] [Google Scholar]
- 63. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. R Development Core Team Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2011. Available at: https://www.r‐project.org/. Accessed November 30, 2020. [Google Scholar]
- 65. GRADEpro . Available at: https://gradepro.org/. Accessed June 18, 2021.
- 66. Adrish M, Chilimuri S, Sun H, Mantri N, Yugay A, Zahid M. The association of renin‐angiotensin‐aldosterone system inhibitors with outcomes among a predominantly ethnic minority patient population hospitalized With COVID‐19: the Bronx experience. Cureus. 2020;12:e10217. doi: 10.7759/cureus.10217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aghaaliakbari F, Abbasi MA, Ranjbar M, Jamshidi Makiani M, Farrokhpour M, Safarnezhad Tameshkel F, Karbalaie Niya MH, Doltkhah S, Yaghoobzadeh K, Savaj S. Angiotensin converting enzyme inhibitors, a risk factor of poor outcome in diabetic patients with COVID‐19 infection. Iran J Kidney Dis. 2020;14:482–487. [PubMed] [Google Scholar]
- 68. Ahlström B, Frithiof R, Hultström M, Larsson I‐M, Strandberg G, Lipcsey M. The Swedish Covid‐19 intensive care cohort: risk factors of ICU admission and ICU mortality. Acta Anaesthesiol Scand. 2021;65:525–533. doi: 10.1111/aas.13781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Akchurin O, Meza K, Biswas S, Greenbaum M, Licona‐Freudenstein AP, Goyal P, Choi JJ, Choi ME. COVID‐19 in patients with CKD in New York City. Kidney360. 2021;2:63–70. doi: 10.34067/KID.0004142020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Akyüz A, Işık F, Aslan B, Çap M, Kaya İ, Atlı Ö, İnci Ü, Taştan E, Aktan A, Bilge Ö, et al. The effect of RAAS inhibitors on acute hypoxemic respiratory failure and in‐hospital mortality in the hypertensive Covid‐19 patients. Clin Exp Hypertens. 2021;43:587–596. doi: 10.1080/10641963.2021.1916947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Alrashed AA, Khan TM, Alhusseini NK, Asdaq SMB, Enani M, Alosaimi B, Alkhani NM, Mohzari Y, Alghalbi MM, Alfahad W, et al. Severity of COVID‐19 infection in ACEI/ARB users in specialty hospitals: a retrospective cohort study. J Infect Public Health. 2021;14:726–733. doi: 10.1016/j.jiph.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. An J, Wei R, Zhou H, Luong TQ, Gould MK, Mefford MT, Harrison TN, Creekmur B, Lee M‐S, Sim JJ, et al. Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers use and COVID‐19 infection among 824 650 patients with hypertension from a US integrated healthcare system. J Am Heart Assoc. 2021;10:e019669. doi: 10.1161/JAHA.120.019669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anzola GP, Bartolaminelli C, Gregorini GA, Coazzoli C, Gatti F, Mora A, Charalampakis D, Palmigiano A, De Simone M, Comini A, et al. No harm from angiotensin‐converting enzyme inhibitors or angiotensin receptor inhibitors in patients with COVID‐19. Results of a prospective study on a hospital‐based cohort. Ital J Med. 2020;14:162–166. doi: 10.4081/itjm.2020.1313 [DOI] [Google Scholar]
- 74. Armstrong K, Soltoff A, Rieu‐Werden M, Metlay J, Haas J. Use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers associated with lower risk of COVID‐19 in household contacts. PLoS One. 2021;16:e0247548. doi: 10.1371/journal.pone.0247548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bae DJ, Tehrani DM, Rabadia SV, Frost M, Parikh RV, Calfon‐Press M, Aksoy O, Umar S, Ardehali R, Rabbani A, et al. Angiotensin converting enzyme inhibitor and angiotensin II receptor blocker use among outpatients diagnosed with COVID‐19. Am J Cardiol. 2020;132:150–157. doi: 10.1016/j.amjcard.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bae JH, Choi SK, Kim NH, Lee J, Kim SG. Use of renin‐angiotensin‐aldosterone system inhibitors and severe COVID‐19 outcomes in patients with hypertension: a nationwide cohort study. Diabetes Metab J. 2021;45:430–438. doi: 10.4093/dmj.2020.0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bae S, Kim JH, Kim Y‐J, Lim JS, Yun S‐C, Kim Y‐H, Lee S‐O, Kim S‐H. Effects of recent use of renin‐angiotensin system inhibitors on mortality of patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7:ofaa519. doi: 10.1093/ofid/ofaa519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Basu A, Agwu JC, Barlow N, Lee B. Hypertension is the major predictor of poor outcomes among inpatients with COVID‐19 infection in the UK: a retrospective cohort study. BMJ Open. 2021;11:e047561. doi: 10.1136/bmjopen-2020-047561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bauer AZ, Gore R, Sama SR, Rosiello R, Garber L, Sundaresan D, McDonald A, Arruda P, Kriebel D. Hypertension, medications, and risk of severe COVID‐19: a Massachusetts community‐based observational study. J Clin Hypertens (Greenwich). 2021;23:21–27. doi: 10.1111/jch.14101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bean DM, Kraljevic Z, Searle T, Bendayan R, Kevin O, Pickles A, Folarin A, Roguski L, Noor K, Shek A, et al. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. Eur J Heart Fail. 2020;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bhattacharyya A, Halder S, Mandal T, Sadhukhan SK, Samajdar SS, Tripathi SK, Pal J, Mukhopadhyay T, Dutta B, Poddar G, et al. Effect of angiotensin converting enzyme inhibitors/angiotensin receptor blockers on COVID‐19 outcome: a record based observational study in West Bengal. J Assoc Physicians India. 2021;69:11–12. [PubMed] [Google Scholar]
- 82. Boari G, Chiarini G, Bonetti S, Malerba P, Bianco G, Faustini C, Braglia‐Orlandini F, Turini D, Guarinoni V, Saottini M, et al. Prognostic factors and predictors of outcome in patients with COVID‐19 and related pneumonia: a retrospective cohort study. Biosci Rep. 2020;40:BSR20203455. doi: 10.1042/BSR20203455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Braude P, Carter B, Short R, Vilches‐Moraga A, Verduri A, Pearce L, Price A, Quinn TJ, Stechman M, Collins J, et al. The influence of ACE inhibitors and ARBs on hospital length of stay and survival in people with COVID‐19. Int J Cardiol Heart Vasc. 2020;31:100660. doi: 10.1016/j.ijcha.2020.100660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bravi F, Flacco ME, Carradori T, Volta CA, Cosenza G, De Togni A, Acuti Martellucci C, Parruti G, Mantovani L, Manzoli L. Predictors of severe or lethal COVID‐19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. PLoS One. 2020;15:e0235248. doi: 10.1371/journal.pone.0235248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Byttebier G, Belmans L, Alexander M, Saxberg BEH, De Spiegeleer B, De Spiegeleer A, Devreker N, Van Praet JT, Vanhove K, Reybrouck R, et al. Hospital mortality in COVID‐19 patients in Belgium treated with statins, ACE inhibitors and/or ARBs. Hum Vaccin Immunother. 2021;17:2841–2850. doi: 10.1080/21645515.2021.1920271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cannata F, Chiarito M, Reimers B, Azzolini E, Ferrante G, My I, Viggiani G, Panico C, Regazzoli D, Ciccarelli M, et al. Continuation versus discontinuation of ACE inhibitors or angiotensin II receptor blockers in COVID‐19: effects on blood pressure control and mortality. Eur Heart J Cardiovasc Pharmacother. 2020;6:412–414. doi: 10.1093/ehjcvp/pvaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cariou B, Hadjadj S, Wargny M, Pichelin M, Al‐Salameh A, Allix I, Amadou C, Arnault G, Baudoux F, Bauduceau B, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chaudhri I, Koraishy FM, Bolotova O, Yoo J, Marcos LA, Taub E, Sahib H, Bloom M, Ahmad S, Skopicki H, et al. Outcomes associated with the use of renin‐angiotensin‐aldosterone system blockade in hospitalized patients with SARS‐CoV‐2 infection. Kidney360. 2020;1:801–809. doi: 10.34067/KID.0003792020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen C, Wang F, Chen P, Jiang J, Cui G, Zhou N, Moroni F, Moslehi JJ, Ammirati E, Wang DW. Mortality and pre‐hospitalization use of renin‐angiotensin system inhibitors in hypertensive COVID‐19 patients. J Am Heart Assoc. 2020;9:e017736. doi: 10.1161/JAHA.120.017736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen F‐F, Zhong M, Liu Y, Zhang Y, Zhang K, Su D‐Z, Meng X, Zhang Y. The characteristics and outcomes of 681 severe cases with COVID‐19 in China. J Crit Care. 2020;60:32–37. doi: 10.1016/j.jcrc.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen R, Yang J, Gao X, Ding X, Yang Y, Shen Y, He C, Xiang H, Ke J, Yuan F, et al. Influence of blood pressure control and application of renin‐angiotensin‐aldosterone system inhibitors on the outcomes in COVID‐19 patients with hypertension. J Clin Hypertens (Greenwich). 2020;22:1974–1983. doi: 10.1111/jch.14038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, Liu C, Xiong M, Deng A, Zhang YU, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660 [DOI] [PubMed] [Google Scholar]
- 93. Cheung KS, Hung IFN, Leung WK. Association between angiotensin blockade and COVID‐19 severity in Hong Kong. CMAJ. 2020;192:E635. doi: 10.1503/cmaj.75865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chodick G, Nutman A, Yiekutiel N, Shalev V. Angiotensin‐converting enzyme inhibitors and angiotensin‐receptor blockers are not associated with increased risk of SARS‐CoV‐2 infection. J Travel Med. 2020;27:taaa069. doi: 10.1093/jtm/taaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Choi MH, Ahn H, Ryu HS, Kim B‐J, Jang J, Jung M, Kim J, Jeong SH. Clinical characteristics and disease progression in early‐stage COVID‐19 patients in South Korea. J Clin Med. 2020;9:1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Christiansen CF, Pottegård A, Heide‐Jørgensen U, Bodilsen J, Søgaard OS, Maeng M, Vistisen ST, Schmidt M, Lund LC, Reilev M, et al. SARS‐CoV‐2 infection and adverse outcomes in users of ACE inhibitors and angiotensin‐receptor blockers: a nationwide case‐control and cohort analysis. Thorax. 2021;76:370–379. doi: 10.1136/thoraxjnl-2020-215768 [DOI] [PubMed] [Google Scholar]
- 97. Chung SM, Lee YY, Ha E, Yoon JS, Won KC, Lee HW, Hur J, Hong KS, Jang JG, Jin HJ, et al. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J. 2020;44:405–413. doi: 10.4093/dmj.2020.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cippà PE, Cugnata F, Ferrari P, Brombin C, Ruinelli L, Bianchi G, Beria N, Schulz L, Bernasconi E, Merlani P, et al. A data‐driven approach to identify risk profiles and protective drugs in COVID‐19. Proc Natl Acad Sci USA. 2021;118. doi: 10.1073/pnas.2016877118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Conversano A, Melillo F, Napolano A, Fominskiy E, Spessot M, Ciceri F, Agricola E. Renin‐angiotensin‐aldosterone system inhibitors and outcome in patients with SARS‐CoV‐2 pneumonia: a case series study. Hypertension. 2020;76:e10–e12. doi: 10.1161/HYPERTENSIONAHA.120.15312 [DOI] [PubMed] [Google Scholar]
- 100. Cordeanu E‐M, Jambert L, Severac F, Lambach H, Tousch J, Heitz M, Mirea C, Hamadé A, Younes W, Frantz A‐S, et al. Outcomes of COVID‐19 hospitalized patients previously treated with renin‐angiotensin system inhibitors. J Clin Med. 2020;9:3472. doi: 10.3390/jcm9113472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Covino M, De Matteis G, Burzo ML, Santoro M, Fuorlo M, Sabia L, Sandroni C, Gasbarrini A, Franceschi F, Gambassi G, et al. Angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers and prognosis of hypertensive patients hospitalised with COVID‐19. Intern Med J. 2020;50:1483–1491. doi: 10.1111/imj.15078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cugno M, Gualtierotti R, Casazza G, Tafuri F, Ghigliazza G, Torri A, Costantino G, Montano N, Peyvandi F. Mortality in patients with COVID‐19 on renin angiotensin system inhibitor long‐term treatment: an observational study showing that things are not always as they seem. Adv Ther. 2021;38:2709–2716. doi: 10.1007/s12325-021-01704-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dalan R, Ang LW, Tan WYT, Fong S‐W, Tay WC, Chan Y‐H, Renia L, Ng LFP, Lye DC, Chew DEK, et al. The association of hypertension and diabetes pharmacotherapy with COVID‐19 severity and immune signatures: an observational study. Eur Heart J Cardiovasc Pharmacother. 2021;7:e48–e51. doi: 10.1093/ehjcvp/pvaa098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, Longhurst CA, Criqui MH, Messer K. Relation of statin use prior to admission to severity and recovery among COVID‐19 inpatients. Am J Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. de Abajo FJ, Rodríguez‐Martín S, Lerma V, Mejía‐Abril G, Aguilar M, García‐Luque A, Laredo L, Laosa O, Centeno‐Soto GA, Ángeles Gálvez M, et al. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. de Abajo FJ, Rodríguez‐Miguel A, Rodríguez‐Martín S, Lerma V, García‐Lledó A, de Abajo FJ, Rodríguez‐Miguel A, Rodríguez‐Martín S, Lerma V, García‐Lledó A, et al.; MED‐ACE2‐COVID19 Study Group . Impact of in‐hospital discontinuation with angiotensin receptor blockers or converting enzyme inhibitors on mortality of COVID‐19 patients: a retrospective cohort study. BMC Med. 2021;19:118. doi: 10.1186/s12916-021-01992-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, Dobson R, Wynendaele E, Van De Wiele C, Vandaele F, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID‐19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21:909–914.e2. doi: 10.1016/j.jamda.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. De Vito A, Geremia N, Princic E, Fanelli C, Panu Napodano CM, Muredda AA, Fiore V, Maida I, Fois AG, Babudieri S, et al. Does Angiotensin II receptor blockers increase the risk of SARS‐CoV‐2 infection? A real‐life experience. Eur Rev Med Pharmacol Sci. 2021;25:523–526. [DOI] [PubMed] [Google Scholar]
- 109. De Vito A, Fiore V, Princic E, Geremia N, Panu Napodano CM, Muredda AA, Maida I, Madeddu G, Babudieri S. Predictors of infection, symptoms development, and mortality in people with SARS‐CoV‐2 living in retirement nursing homes. PLoS One. 2021;16:e0248009. doi: 10.1371/journal.pone.0248009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Desai A, Voza G, Paiardi S, Teofilo FI, Caltagirone G, Pons MR, Aloise M, Kogan M, Tommasini T, Savevski V, et al. The role of anti‐hypertensive treatment, comorbidities and early introduction of LMWH in the setting of COVID‐19: a retrospective, observational study in Northern Italy. Int J Cardiol. 2021;324:249–254. doi: 10.1016/j.ijcard.2020.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Díaz‐Guardiola P, Martín‐Borge V, García‐Fernández C, Ramírez‐Prieto MT, Ramírez‐Belmar MI, García‐Romero G, de la Calle E, Balsa JA. Association of use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with coronavirus disease 2019 severity and mortality. Am J Intern Med. 2020;8:204. doi: 10.11648/j.ajim.20200805.12 [DOI] [Google Scholar]
- 112. Dublin S, Walker RL, Floyd JS, Shortreed SM, Fuller S, Albertson‐Junkans L, Harrington LB, Greenwood‐Hickman MA, Green BB, Psaty BM. Renin‐angiotensin‐aldosterone system inhibitors and COVID‐19 infection or hospitalization: a cohort study. Am J Hypertens. 2021;34:339–347. doi: 10.1093/ajh/hpaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. El‐Battrawy I, Nuñez‐Gil IJ, Abumayyaleh M, Estrada V, Manuel Becerra‐Muñoz V, Uribarri A, Fernández‐Rozas I, Feltes G, Arroyo‐Espliguero R, Trabattoni D, et al. COVID‐19 and the impact of arterial hypertension—an analysis of the international HOPE COVID‐19 Registry (Italy‐Spain‐Germany). Eur J Clin Invest. 2021;51:e13582. doi: 10.1111/eci.13582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. ElAbd R, AlTarrah D, AlYouha S, Bastaki H, Almazeedi S, Al‐Haddad M, Jamal M, AlSabah S. Angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) are protective against ICU admission and mortality for patients with COVID‐19 disease. Front Med (Lausanne). 2021;8:600385. doi: 10.3389/fmed.2021.600385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Felice C, Nardin C, Di Tanna GL, Grossi U, Bernardi E, Scaldaferri L, Romagnoli M, Tonon L, Cavasin P, Novello S, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID‐19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. 2020;33:944–948. doi: 10.1093/ajh/hpaa096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Feng Z, Li J, Yao S, Yu Q, Zhou W, Mao X, Li H, Kang W, Ouyang X, Mei JI, et al. Clinical factors associated with progression and prolonged viral shedding in COVID‐19 patients: a multicenter study. Aging Dis. 2020;11:1069–1081. doi: 10.14336/AD.2020.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ferrante G, Fazzari F, Cozzi O, Maurina M, Bragato R, D’Orazio F, Torrisi C, Lanza E, Indolfi E, Donghi V, et al. Risk factors for myocardial injury and death in patients with COVID‐19: insights from a cohort study with chest computed tomography. Cardiovasc Res. 2020;116:2239–2246. doi: 10.1093/cvr/cvaa193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fosbøl EL, Butt JH, Østergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324:168–177. doi: 10.1001/jama.2020.11301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fröhlich GM, Jeschke E, Eichler U, Thiele H, Alhariri L, Reinthaler M, Kastrati A, Leistner DM, Skurk C, Landmesser U, et al. Impact of oral anticoagulation on clinical outcomes of COVID‐19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol. 2021;110:1041–1050. doi: 10.1007/s00392-020-01783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, Li QI, Li W, Yang S, Zhao X, et al. Association of hypertension and antihypertensive treatment with COVID‐19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gaspar P, Parreira I, Antunes Meireles P, Bessa F, Dias Silva V, Abrantes AM, Pais de Lacerda A, Mota C. The effect of chronic and inhospital exposure to renin‐angiotensin system inhibitors on the outcome and inflammatory state of coronavirus disease 2019 adult inpatients. Int J Hypertens. 2021;2021:5517441. doi: 10.1155/2021/5517441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gault N, Esposito‐Farèse M, Revest M, Inamo J, Cabié A, Polard É, Hulot J‐S, Ghosn J, Chirouze C, Deconinck L, et al. Chronic use of renin‐angiotensin‐aldosterone system blockers and mortality in COVID‐19: a multicenter prospective cohort and literature review. Fundam Clin Pharmacol. 2021;35:1141–1158. doi: 10.1111/fcp.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Genet B, Vidal J‐S, Cohen A, Boully C, Beunardeau M, Marine Harlé L, Gonçalves A, Boudali Y, Hernandorena I, Bailly H, et al. COVID‐19 In‐hospital mortality and use of renin‐angiotensin system blockers in geriatrics patients. J Am Med Dir Assoc. 2020;21:1539–1545. doi: 10.1016/j.jamda.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Georges J‐L, Gilles F, Cochet H, Bertrand A, De Tournemire M, Monguillon V, Pasqualini M, Prevot A, Roger G, Saba J, et al. Positive association of angiotensin II receptor blockers, not angiotensin‐converting enzyme inhibitors, with an increased vulnerability to SARS‐CoV‐2 infection in patients hospitalized for suspected COVID‐19 pneumonia. PLoS One. 2020;15:e0244349. doi: 10.1371/journal.pone.0244349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R; Reggio Emilia COVID‐19 Working Group . Characteristics and outcomes of a cohort of COVID‐19 patients in the Province of Reggio Emilia, Italy. PLoS One. 2020;15:e0238281. doi: 10.1371/journal.pone.0238281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gnavi R, Demaria M, Picariello R, Dalmasso M, Ricceri F, Costa G. Therapy with agents acting on the renin‐angiotensin system and risk of severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. 2020;71:2291–2293. doi: 10.1093/cid/ciaa634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gobbato M, Calagnan E, Burba I, Rizzi L, Grassetti L, Del Zotto S, Dal Maso L, Serraiono D, Tonutti G. Clinical, demographical characteristics and hospitalisation of 3,010 patients with Covid‐19 in Friuli Venezia Giulia Region (Northern Italy). A multivariate, population‐based, statistical analysis. Epidemiol Prev. 2020;44:226–234. [DOI] [PubMed] [Google Scholar]
- 128. Golpe R, Pérez‐de‐Llano LA, Dacal D, Guerrero‐Sande H, Pombo‐Vide B, Ventura‐Valcárcel P; Lugo Covid‐19 team . Risk of severe COVID‐19 in hypertensive patients treated with renin‐angiotensin‐aldosterone system inhibitors. Med Clin (Engl Ed). 2020;155:488–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gormez S, Ekicibasi E, Degirmencioglu A, Paudel A, Erdim R, Gumusel HK, Eroglu E, Tanboga IH, Dagdelen S, Sariguzel N, et al. Association between renin‐angiotensin‐aldosterone system inhibitor treatment, neutrophil‐lymphocyte ratio, D‐Dimer and clinical severity of COVID‐19 in hospitalized patients: a multicenter, observational study. J Hum Hypertens. 2021;35:588–597. doi: 10.1038/s41371-020-00405-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Graham DJ, Izurieta HS, Muthuri SG, Zhang DI, Sandhu AT, Lu Y, Zhao Y, Feng Y, Eworuke E, Lyu H, et al. Risk of Covid‐19‐related hospitalization and more severe outcomes in medicare beneficiaries treated with renin‐angiotensin‐aldosterone system inhibitors for hypertension. J Gen Intern Med. 2021;36:3802–3809. doi: 10.1007/s11606-021-07155-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gupta R, Agrawal R, Bukhari Z, Jabbar A, Wang D, Diks J, Alshal M, Emechebe DY, Brunicardi FC, Lazar JM, et al. Higher comorbidities and early death in hospitalized African‐American patients with Covid‐19. BMC Infect Dis. 2021;21:78. doi: 10.1186/s12879-021-05782-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hakeam HA, Alsemari M, Duhailib ZA, Ghonem L, Alharbi SA, Almutairy E, Sheraim NMB, Alsalhi M, Alhijji A, AlQahtani S, et al. Association of angiotensin‐converting enzyme inhibitors and angiotensin II blockers with severity of COVID‐19: a multicenter, prospective study. J Cardiovasc Pharmacol Ther. 2020;26:244–252. doi: 10.1177/1074248420976279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Haroon S, Subramanian A, Cooper J, Anand A, Gokhale K, Byne N, Dhalla S, Acosta‐Mena D, Taverner T, Okoth K, et al. Renin‐angiotensin system inhibitors and susceptibility to COVID‐19 in patients with hypertension: a propensity score‐matched cohort study in primary care. BMC Infect Dis. 2021;21:262. doi: 10.1186/s12879-021-05951-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hippisley‐Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, Aveyard P, Pavord ID, Watkinson PJ. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Holt A, Mizrak I, Lamberts M, Lav MP. Influence of inhibitors of the renin‐angiotensin system on risk of acute respiratory distress syndrome in Danish hospitalized COVID‐19 patients. J Hypertens. 2020;38:1612–1613. doi: 10.1097/HJH.0000000000002515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M, Cicero AFG, Minuz P, Muiesan ML, Mulatero P, et al. Age and multimorbidity predict death among COVID‐19 patients: results of the SARS‐RAS study of the Italian Society of Hypertension. Hypertension. 2020;76:366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324 [DOI] [PubMed] [Google Scholar]
- 138. Imam Z, Odish F, Gill I, O’Connor D, Armstrong J, Vanood A, Ibironke O, Hanna A, Ranski A, Halalau A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID‐19 patients in Michigan, United States. J Intern Med. 2020;288:469–476. doi: 10.1111/joim.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jung C, Bruno RR, Wernly B, Joannidis M, Oeyen S, Zafeiridis T, Marsh B, Andersen FH, Moreno R, Fernandes AM, et al. Inhibitors of the renin‐angiotensin‐aldosterone system and COVID‐19 in critically ill elderly patients. Eur Heart J Cardiovasc Pharmacother. 2021;7:76–77. doi: 10.1093/ehjcvp/pvaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jung S‐Y, Choi JC, You S‐H, Kim W‐Y. Association of renin‐angiotensin‐aldosterone system inhibitors with coronavirus disease 2019 (COVID‐19)‐ related outcomes in Korea: a nationwide population‐based cohort study. Clin Infect Dis. 2020;71:2121–2128. doi: 10.1093/cid/ciaa624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Jurado A, Martín MC, Abad‐Molina C, Orduña A, Martínez A, Ocaña E, Yarce O, Navas AM, Trujillo A, Fernández L, et al. COVID‐19: age, interleukin‐6, C‐reactive protein, and lymphocytes as key clues from a multicentre retrospective study. Immun Ageing. 2020;17:22. doi: 10.1186/s12979-020-00194-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kang S‐H, Lee D‐H, Han K‐D, Jung J‐H, Park S‐H, Dai AM, Wei HG, Yoon C‐H, Youn T‐J, Chae I‐H, et al. Hypertension, renin‐angiotensin‐aldosterone‐system‐blocking agents, and COVID‐19. Clin Hypertens. 2021;27:11. doi: 10.1186/s40885-021-00168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Khan KS, Reed‐Embleton H, Lewis J, Bain P, Mahmud S. Angiotensin converting enzyme inhibitors do not increase the risk of poor outcomes in COVID‐19 disease. A multi‐centre observational study. Scott Med J. 2020;65:149–153. doi: 10.1177/0036933020951926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Khera R, Clark C, Lu Y, Guo Y, Ren S, Truax B, Spatz ES, Murugiah K, Lin Z, Omer SB, et al. Association of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease‐19. J Am Heart Assoc. 2021;10:e018086. doi: 10.1161/JAHA.120.018086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kim E, Kim YC, Park JY, Jung J, Lee JP, Kim H. Evaluation of the prognosis of COVID‐19 patients according to the presence of underlying diseases and drug treatment. Int J Environ Res Public Health. 2021;18:5342. doi: 10.3390/ijerph18105342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kim H‐S, Kang M, Kang G. Renin‐angiotensin system modulators and other risk factors in COVID‐19 patients with hypertension: a Korean perspective. BMC Infect Dis. 2021;21:175. doi: 10.1186/s12879-021-05848-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kim J, Kim DW, Kim KI, Kim HB, Kim JH, Lee YG, Byeon KH, Cheong HK; Korean Society of Hypertension . Compliance of antihypertensive medication and risk of coronavirus disease 2019: a cohort study using big data from the Korean National Health Insurance Service. J Korean Med Sci. 2020;35:e232. doi: 10.3346/jkms.2020.35.e232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kim JH, Baek Y‐H, Lee H, Choe YJ, Shin HJ, Shin J‐Y. Clinical outcomes of COVID‐19 following the use of angiotensin‐converting enzyme inhibitors or angiotensin‐receptor blockers among patients with hypertension in Korea: a nationwide study. Epidemiol Health. 2021;43:e2021004. doi: 10.4178/epih.e2021004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kim MK, Jeon J‐H, Kim S‐W, Moon JS, Cho NH, Han E, You JH, Lee JY, Hyun M, Park JS, et al. The clinical characteristics and outcomes of patients with moderate‐to‐severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J. 2020;44:602–613. doi: 10.4093/dmj.2020.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kocayigit I, Kocayigit H, Yaylaci S, Can Y, Erdem AF, Karabay O. Impact of antihypertensive agents on clinical course and in‐hospital mortality: analysis of 169 hypertensive patients hospitalized for COVID‐19. Rev Assoc Med Bras (1992). 2020;66(suppl 2):71–76. doi: 10.1590/1806-9282.66.s2.71 [DOI] [PubMed] [Google Scholar]
- 151. Kolin DA, Kulm S, Christos PJ, Elemento O. Clinical, regional, and genetic characteristics of Covid‐19 patients from UK Biobank. PLoS One. 2020;15:e0241264. doi: 10.1371/journal.pone.0241264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. LaClair HJ, Khosrodad N, Sule AA, Koehler T, Krishnamoorthy G. Effect of ACE inhibitors and angiotensin receptor blockers on in‐hospital mortality and length of stay in hospitalized COVID‐19 patients. Vascul Pharmacol. 2021;141:106902. doi: 10.1016/j.vph.2021.106902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lafaurie M, Martin‐Blondel G, Delobel P, Charpentier S, Sommet A, Moulis G. Outcome of patients hospitalized for COVID‐19 and exposure to angiotensin‐converting enzyme inhibitors and angiotensin‐receptor blockers in France: results of the ACE‐CoV study. Fundam Clin Pharmacol. 2021;35:194–203. doi: 10.1111/fcp.12613 [DOI] [PubMed] [Google Scholar]
- 154. Lahens A, Mullaert J, Gressens S, Gault N, Flamant M, Deconinck L, Joly V, Yazdanpanah Y, Lescure F‐X, Vidal‐Petiot E. Association between renin‐angiotensin‐aldosterone system blockers and outcome in coronavirus disease 2019: analysing in‐hospital exposure generates a biased seemingly protective effect of treatment. J Hypertens. 2021;39:367–375. doi: 10.1097/HJH.0000000000002658 [DOI] [PubMed] [Google Scholar]
- 155. Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, Mallipattu SK, Skopicki HA, Singer AJ, Duong TQ. Continued in‐hospital angiotensin‐converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID‐19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222:1256–1264. doi: 10.1093/infdis/jiaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lee H‐Y, Ahn J, Park J, Kang CK, Won S‐H, Kim DW, Park J‐H, Chung K‐H, Joh J‐S, Bang JIH, et al. Different therapeutic associations of renin‐angiotensin system inhibitors with coronavirus disease 2019 compared with usual pneumonia. Korean J Intern Med. 2021;36:617–628. doi: 10.3904/kjim.2020.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lee J, Jo SJ, Cho Y, Lee JH, Oh I‐Y, Park JJ, Cho Y‐S, Choi D‐J. Effects of renin‐angiotensin system blockers on the risk and outcomes of severe acute respiratory syndrome coronavirus 2 infection in patients with hypertension. Korean J Intern Med. 2021;36:S123–S131. doi: 10.3904/kjim.2020.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Li M, Wang Y, Ndiwane N, Orner MB, Palacios N, Mittler B, Berlowitz D, Kazis LE, Xia W. The association of COVID‐19 occurrence and severity with the use of angiotensin converting enzyme inhibitors or angiotensin‐II receptor blockers in patients with hypertension. PLoS One. 2021;16:e0248652. doi: 10.1371/journal.pone.0248652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li S, Sarangarajan R, Jun T, Kao Y‐H, Wang Z, Hao KE, Schadt E, Kiebish MA, Granger E, Narain NR, et al. In‐hospital use of ACE inhibitors/angiotensin receptor blockers associates with COVID‐19 outcomes in African American patients. J Clin Invest. 2021;131:e151418. doi: 10.1172/JCI151418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Liabeuf S, Moragny J, Bennis Y, Batteux B, Brochot E, Schmit JL, Lanoix J‐P, Andrejak C, Ganry O, Slama M, et al. Association between renin‐angiotensin system inhibitors and COVID‐19 complications. Eur Heart J Cardiovasc Pharmacother. 2021;7:426–434. doi: 10.1093/ehjcvp/pvaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lim J‐H, Cho J‐H, Jeon Y, Kim JH, Lee GY, Jeon S, Noh HW, Lee Y‐H, Lee J, Chang H‐H, et al. Adverse impact of renin‐angiotensin system blockade on the clinical course in hospitalized patients with severe COVID‐19: a retrospective cohort study. Sci Rep. 2020;10:20250. doi: 10.1038/s41598-020-76915-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Liu X, Liu Y, Chen K, Yan S, Bai X, Li J, Liu D. Efficacy of ACEIs/ARBs vs CCBs on the progression of COVID‐19 patients with hypertension in Wuhan: a hospital‐based retrospective cohort study. J Med Virol. 2021;93:854–862. doi: 10.1002/jmv.26315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Loader J, Lampa E, Gustafsson S, Cars T, Sundström J. Renin‐angiotensin aldosterone system inhibitors in primary prevention and COVID‐19. J Am Heart Assoc. 2021;10:e021154. doi: 10.1161/JAHA.120.021154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. López‐Otero D, López‐Pais J, Cacho‐Antonio CE, Antúnez‐Muiños PJ, González‐Ferrero T, Pérez‐Poza M, Otero‐García Ó, Díaz‐Fernández B, Bastos‐Fernández M, Bouzas‐Cruz N, et al. Impact of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on COVID‐19 in a western population. CARDIOVID registry. Rev Esp Cardiol (Engl Ed). 2021;74:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lozano‐Montoya I, Quezada‐Feijoo M, Jaramillo‐Hidalgo J, Garmendia‐Prieto B, Lisette‐Carrillo P, Gómez‐Pavón FJ. Mortality risk factors in a Spanish cohort of oldest‐old patients hospitalized with COVID‐19 in an acute geriatric unit: the OCTA‐COVID study. Eur Geriatr Med. 2021;12:1169–1180. doi: 10.1007/s41999-021-00541-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ma Y, Zhang Y, Li S, Yang H, Li H, Cao Z, Xu F, Sun L, Wang Y. Sex differences in association between anti‐hypertensive medications and risk of COVID‐19 in middle‐aged and older adults. Drugs Aging. 2021;38:921–930. doi: 10.1007/s40266-021-00886-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Martínez‐Botía P, Bernardo Á, Acebes‐Huerta A, Caro A, Leoz B, Martínez‐Carballeira D, Palomo‐Antequera C, Soto I, Gutiérrez L. Clinical management of hypertension, inflammation and thrombosis in hospitalized COVID‐19 patients: impact on survival and concerns. J Clin Med. 2021;10:1073. doi: 10.3390/jcm10051073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Martínez‐Del Río J, Piqueras‐Flores J, Martín N‐S, de la Sierra P, Negreira‐Caamaño M, Águila‐Gordo D, Mateo‐Gómez C, Salas‐Bravo D, Rodríguez‐Martínez M. Comparative analysis between the use of renin‐angiotensin system antagonists and clinical outcomes of hospitalized patients with COVID‐19 respiratory infection. Med Clin (Engl Ed). 2020;155:473–481. doi: 10.1016/j.medcle.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mayer MA, Vidal‐Alaball J, Puigdellívol‐Sánchez A, Marín Gomez FX, Leis A, Mendioroz PJ. Clinical characterization of patients with COVID‐19 in primary care in Catalonia: retrospective observational study. JMIR Public Health Surveill. 2021;7:e25452. doi: 10.2196/25452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona‐Rubio AE, Jacob M, Procop GW, Harrington S, et al. Association of use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Meng X, Liu Y, Wei C, Zhang K, Zhang Y, Zhong M, Zhang C, Zhang Y. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers improved the outcome of patients with severe COVID‐19 and hypertension. Sci China Life Sci. 2021;64:836–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Million M, Lagier J‐C, Gautret P, Colson P, Fournier P‐E, Amrane S, Hocquart M, Mailhe M, Esteves‐Vieira V, Doudier B, et al. Early treatment of COVID‐19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Morales DR, Conover MM, You SC, Pratt N, Kostka K, Duarte‐Salles T, Fernández‐Bertolín S, Aragón M, DuVall SL, Lynch K, et al. Renin‐angiotensin system blockers and susceptibility to COVID‐19: an international, open science, cohort analysis. Lancet Digit Health. 2021;3:e98–e114. doi: 10.1016/S2589-7500(20)30289-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mostaza JM, García‐Iglesias F, González‐Alegre T, Blanco F, Varas M, Hernández‐Blanco C, Hontañón V, Jaras‐Hernández MJ, Martínez‐Prieto M, Menéndez‐Saldaña A, et al. Clinical course and prognostic factors of COVID‐19 infection in an elderly hospitalized population. Arch Gerontol Geriatr. 2020;91:104204. doi: 10.1016/j.archger.2020.104204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Mustafic H, Fayssoil A, Josseran L, Ouadahi M, Grimaldi‐Bensouda L, Dubourg O, Annane D, Mansencal N. Impact of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers in hypertensive patients with COVID‐19 (COVIDECA Study). Am J Cardiol. 2021;147:58–60. doi: 10.1016/j.amjcard.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Negreira‐Caamaño M, Piqueras‐Flores J, Martínez‐DelRio J, Nieto‐Sandoval‐Martin‐DeLaSierra P, Aguila‐Gordo D, Mateo‐Gomez C, Salas‐Bravo D, Rodriguez‐Martinez M, Negreira‐Caamaño M. Impact of treatment with renin‐angiotensin system inhibitors on clinical outcomes in hypertensive patients hospitalized with COVID‐19. High Blood Press Cardiovasc Prev. 2020;27:561–568. doi: 10.1007/s40292-020-00409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ng JH, Hirsch JS, Wanchoo R, Sachdeva M, Sakhiya V, Hong S, Jhaveri KD, Fishbane S, Abate M, Andrade HP, et al.; Northwell COVID‐19 Research Consortium and the Northwell Nephrology COVID‐19 Research Consortium . Outcomes of patients with end‐stage kidney disease hospitalized with COVID‐19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Núñez‐Gil IJ, Olier I, Feltes G, Viana‐Llamas MC, Maroun‐Eid C, Romero R, Fernández‐Rozas I, Uribarri A, Becerra‐Muñoz VM, Alfonso‐Rodriguez E, et al. Renin‐angiotensin system inhibitors effect before and during hospitalization in COVID‐19 outcomes: final analysis of the international HOPE COVID‐19 (Health Outcome Predictive Evaluation for COVID‐19) registry. Am Heart J. 2021;237:104–115. doi: 10.1016/j.ahj.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Núñez‐Gil IJ, Fernández‐Ortiz A, Maroud Eid C, Huang J, Romero R, Becerra‐Muñoz VM, Uribarri A, Feltes G, Trabatoni D, Fernandez‐Rozas I, et al. Underlying heart diseases and acute COVID‐19 outcomes. Cardiol Journal. 2021;28:202–214. doi: 10.5603/CJ.a2020.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Oliveira J, Gameiro J, Bernardo J, Marques F, Costa C, Branco C, Duarte I, Fonseca J, Carreiro C, Braz S, et al. Impact of chronic RAAS use in elderly COVID‐19 patients: a retrospective analysis. J Clin Med. 2021;10:3147. doi: 10.3390/jcm10143147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Palazzuoli A, Mancone M, De Ferrari GM, Forleo G, Secco GG, Ruocco GM, D'Ascenzo F, Monticone S, Paggi A, Vicenzi M, et al. Antecedent administration of angiotensin‐converting enzyme inhibitors or angiotensin II receptor antagonists and survival after hospitalization for COVID‐19 syndrome. J Am Heart Assoc. 2020;9:e017364. doi: 10.1161/JAHA.120.017364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Park J, Lee S‐H, You SC, Kim J, Yang K. Effect of renin‐angiotensin‐aldosterone system inhibitors on Covid‐19 patients in Korea. PLoS One. 2021;16:e0248058. doi: 10.1371/journal.pone.0248058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Peñalvo JL, Genbrugge E, Mertens E, Sagastume D, van der Sande MAB, Widdowson M‐A, Van Beckhoven D. Insights into the association of ACEIs/ARBs use and COVID‐19 prognosis: a multistate modelling study of nationwide hospital surveillance data from Belgium. BMJ Open. 2021;11:e053393. doi: 10.1136/bmjopen-2021-053393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Peng C, Wang H, Guo Y‐F, Qi G‐Y, Zhang C‐X, Chen T, He J, Jin Z‐C. Calcium channel blockers improve prognosis of patients with coronavirus disease 2019 and hypertension. Chin Med J (Engl). 2021;134:1602–1609. doi: 10.1097/CM9.0000000000001479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Raisi‐Estabragh Z, McCracken C, Ardissino M, Bethell MS, Cooper J, Cooper C, Harvey NC, Petersen SE. Renin‐angiotensin‐aldosterone system blockers are not associated with coronavirus disease 2019 (COVID‐19) hospitalization: study of 1,439 UK Biobank cases. Front Cardiovasc Med. 2020;7:138. doi: 10.3389/fcvm.2020.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ramos‐Rincón JM, Pérez‐Belmonte LM, Carrasco‐Sánchez FJ, Jansen‐Chaparro S, De‐Sousa‐Baena M, Bueno‐Fonseca J, Pérez‐Aguilar M, Arévalo‐Cañas C, Bacete Cebrian M, Méndez‐Bailón M, et al. Cardiometabolic therapy and mortality in very old patients with diabetes hospitalized due to COVID‐19. J Gerontol A Biol Sci Med Sci. 2021;76:e102–e109. doi: 10.1093/gerona/glab124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Ran J, Song Y, Zhuang Z, Han L, Zhao S, Cao P, Geng Y, Xu L, Qin J, He D, et al. Blood pressure control and adverse outcomes of COVID‐19 infection in patients with concomitant hypertension in Wuhan, China. Hypertens Res. 2020;43:1267–1276. doi: 10.1038/s41440-020-00541-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Rezel‐Potts E, Douiri A, Chowienczyk PJ, Gulliford MC. Antihypertensive medications and COVID‐19 diagnosis and mortality: population‐based case‐control analysis in the United Kingdom. Br J Clin Pharmacol. 2021;87:4598–4607. doi: 10.1111/bcp.14873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Rodilla E, Saura A, Jiménez I, Mendizábal A, Pineda‐Cantero A, Lorenzo‐Hernández E, Fidalgo‐Montero MDP, López‐Cuervo JF, Gil‐Sánchez R, Rabadán‐Pejenaute E, et al. Association of hypertension with all‐cause mortality among hospitalized patients with COVID‐19. J Clin Med. 2020;9:3136. doi: 10.3390/jcm9103136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in‐hospital mortality in a US national sample of patients with COVID‐19. JAMA Netw Open. 2020;3:e2029058. doi: 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rossi L, Biagi A, Zanni A, Sticozzi C, Comastri G, Villani GQ, Malagoli A, Pannone L, Vergara P, Gandolfi S. Renin‐angiotensin system inhibitors and mortality in patients with COVID‐19. Infection. 2021;49:287–294. doi: 10.1007/s15010-020-01550-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Roy‐Vallejo E, Sánchez Purificación A, Torres Peña J, Sánchez Moreno B, Arnalich F, García Blanco M, López Miranda J, Romero‐Cabrera J, Herrero Gil C, Bascunana J, et al. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers withdrawal is associated with higher mortality in hospitalized patients with COVID‐19. J Clin Med. 2021;10:2642. doi: 10.3390/jcm10122642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rubin SJS, Falkson SR, Degner NR, Blish CA. Safety of ACE‐I and ARB medications in COVID‐19: a retrospective cohort study of inpatients and outpatients in California. J Clin Transl Sci. 2021;5:e8. doi: 10.1017/cts.2020.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Sablerolles RSG, Hogenhuis FEF, Lafeber M, van de Loo BPA, Borgsteede SD, Boersma E, Versmissen J, van der Kuy H. No association between use of angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers prior to hospital admission and clinical course of COVID‐19 in the COvid MEdicaTion (COMET) study. Br J Clin Pharmacol. 2021;87:3301–3309. doi: 10.1111/bcp.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Sandhu AT, Kohsaka S, Lin S, Woo CY, Goldstein MK, Heidenreich PA. Renin–angiotensin–aldosterone system inhibitors and SARS‐CoV‐2 infection: an analysis from the Veteran’s Affairs Healthcare System. Am Heart J. 2021;240:46–57. doi: 10.1016/j.ahj.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Satué‐Gracia EM, Vila‐Córcoles A, de Diego‐Cabanes C, Vila‐Rovira A, Torrente‐Fraga C, Gómez‐Bertomeu F, Hospital‐Guardiola I, Ochoa‐Gondar O, Martín‐Luján F. Susceptibility and risk of SARS‐COV‐2 infection among middle‐aged and older adults in Tarragona area, Spain. Med Clin (Barc). 2022;158:251–259. doi: 10.1016/j.medcli.2021.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Savarese G, Benson L, Sundström J, Lund LH. Association between renin‐angiotensin‐aldosterone system inhibitor use and COVID‐19 hospitalization and death: a 1.4 million patient nationwide registry analysis. Eur J Heart Fail. 2021;23:476–485. doi: 10.1002/ejhf.2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Selçuk M, Çınar T, Keskin M, Çiçek V, Kılıç Ş, Kenan B, Doğan S, Asal S, Günay N, Yıldırım E, et al. Is the use of ACE inb/ARBs associated with higher in‐hospital mortality in Covid‐19 pneumonia patients? Clin Exp Hypertens. 2020;42:738–742. doi: 10.1080/10641963.2020.1783549 [DOI] [PubMed] [Google Scholar]
- 201. Semenzato L, Botton J, Drouin J, Baricault B, Vabre C, Cuenot F, Penso L, Herlemont P, Sbidian E, Weill A, et al. Antihypertensive drugs and COVID‐19 risk: a cohort study of 2 million hypertensive patients. Hypertension. 2021;77:833–842. doi: 10.1161/HYPERTENSIONAHA.120.16314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Şenkal N, Meral R, Medetalibeyoğlu A, Konyaoğlu H, Kose M, Tukek T. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID‐19. Anatol J Cardiol. 2020;24:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Seo J, Son M. Update on association between exposure to renin‐angiotensin‐aldosterone system inhibitors and coronavirus disease 2019 in South Korea. Korean J Intern Med. 2021;36:S114–S122. doi: 10.3904/kjim.2020.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Shaghee F, Nafakhi H, Al‐Buthabhak K, Alareedh M, Nafakhi A, Kasim S. Blood parameters, symptoms at presentation and adverse in‐hospital outcomes of COVID‐19 pneumonia in patients with hypertension. Arter Hypertens. 2021;25:7–14. doi: 10.5603/AH.a2021.0004 [DOI] [Google Scholar]
- 205. Shah P, Owens J, Franklin J, Jani Y, Kumar A, Doshi R. Baseline use of angiotensin‐converting enzyme inhibitor/AT1 blocker and outcomes in hospitalized coronavirus disease 2019 African‐American patients. J Hypertens. 2020;38:2537–2541. doi: 10.1097/HJH.0000000000002584 [DOI] [PubMed] [Google Scholar]
- 206. Soleimani A, Kazemian S, Karbalai Saleh S, Aminorroaya A, Shajari Z, Hadadi A, Talebpour M, Sadeghian H, Payandemehr P, Sotoodehnia M, et al. Effects of angiotensin receptor blockers (ARBs) on in‐hospital outcomes of patients with hypertension and confirmed or clinically suspected COVID‐19. Am J Hypertens. 2020;33:1102–1111. doi: 10.1093/ajh/hpaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Soler MJ, Noordzij M, Abramowicz D, de Arriba G, Basile C, van Buren M, Covic A, Crespo M, Duivenvoorden R, Massy ZA, et al. Renin‐angiotensin system blockers and the risk of COVID‐19–related mortality in patients with kidney failure. Clin J Am Soc Nephrol. 2021;16:1061–1072. doi: 10.2215/CJN.18961220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Son M, Seo J, Yang S. Association between renin‐angiotensin‐aldosterone system inhibitors and COVID‐19 infection in South Korea. Hypertension. 2020;76:742–749. doi: 10.1161/HYPERTENSIONAHA.120.15464 [DOI] [PubMed] [Google Scholar]
- 209. Stevens JS, King KL, Robbins‐Juarez SY, Khairallah P, Toma K, Alvarado Verduzco H, Daniel E, Douglas D, Moses AA, Peleg Y, et al. High rate of renal recovery in survivors of COVID‐19 associated acute renal failure requiring renal replacement therapy. PLoS One. 2020;15:e0244131. doi: 10.1371/journal.pone.0244131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Tavolinejad H, Hosseini K, Sadeghian S, Pourhosseini H, Lotfi‐Tokaldany M, Masoudkabir F, Sattartabar B, Masoudi M, Shafiee A, Badalabadi RM, et al. Clinical implications and indicators of mortality among patients hospitalized with concurrent COVID‐19 and myocardial infarction. Turk Kardiyol Dern Ars. 2021;49:293–302. doi: 10.5543/tkda.2021.14331 [DOI] [PubMed] [Google Scholar]
- 211. Tedeschi S, Giannella M, Bartoletti M, Trapani F, Tadolini M, Borghi C, Viale P. Clinical impact of renin‐angiotensin system inhibitors on in‐hospital mortality of patients with hypertension hospitalized for coronavirus disease 2019. Clin Infect Dis. 2020;71:899–901. doi: 10.1093/cid/ciaa492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Terlecki M, Wojciechowska W, Klocek M, Olszanecka A, Stolarz‐Skrzypek K, Grodzicki T, Małecki M, Katra B, Garlicki A, Bociąga‐Jasik M, et al. Association between cardiovascular disease, cardiovascular drug therapy, and in‐hospital outcomes in patients with COVID‐19: data from a large single‐center registry in Poland. Kardiol Pol. 2021;79:773–780. doi: 10.33963/KP.15990 [DOI] [PubMed] [Google Scholar]
- 213. Tetlow S, Segiet‐Swiecicka A, O’Sullivan R, O’Halloran S, Kalb K, Brathwaite‐Shirley C, Alger L, Ankuli A, Baig MS, Catmur F, et al. ACE inhibitors, angiotensin receptor blockers and endothelial injury in COVID‐19. J Intern Med. 2021;289:688–699. doi: 10.1111/joim.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Trifirò G, Massari M, Da Cas R, Menniti Ippolito F, Sultana J, Crisafulli S, Giorgi Rossi P, Marino M, Zorzi M, Bovo E, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of death in patients hospitalised with COVID‐19: a retrospective Italian Cohort Study of 43,000 patients. Drug Saf. 2020;43:1297–1308. doi: 10.1007/s40264-020-00994-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Trump S, Thurmann L, Loske J, Messingschlager M, Lukassen S, Chua RL, Liebig J, Krieger T, Hennig BP, Twardziok S, et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID‐19. Nat Biotechnol. 2021;39:705–716. [DOI] [PubMed] [Google Scholar]
- 216. Tse G, Zhou J, Lee S, Wong WT, Li X, Liu T, Cao Z, Zeng DD, Wai AKC, Wong ICK, et al. Relationship between angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and COVID‐19 incidence or severe disease. J Hypertens. 2021;39:1717–1724. doi: 10.1097/HJH.0000000000002866 [DOI] [PubMed] [Google Scholar]
- 217. Vera M, Kattan E, Born P, Rivas E, Amthauer M, Nesvadba A, Lara B, Rao I, Espíndola E, Rojas L, et al. Intubation timing as determinant of outcome in patients with acute respiratory distress syndrome by SARS‐CoV‐2 infection. J Crit Care. 2021;65:164–169. doi: 10.1016/j.jcrc.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Vila‐Córcoles A, Ochoa‐Gondar O, Satué‐Gracia EM, Torrente‐Fraga C, Gomez‐Bertomeu F, Vila‐Rovira A, Hospital‐Guardiola I, de Diego‐Cabanes C, Bejarano‐Romero F, Basora‐Gallisà J. Influence of prior comorbidities and chronic medications use on the risk of COVID‐19 in adults: a population‐based cohort study in Tarragona, Spain. BMJ Open. 2020;10:e041577. doi: 10.1136/bmjopen-2020-041577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Vila‐Corcoles A, Satue‐Gracia E, Ochoa‐Gondar O, Torrente‐Fraga C, Gomez‐Bertomeu F, Vila‐Rovira A, Hospital‐Guardiola I, Diego‐Cabanes C, Bejarano‐Romero F, Rovira‐Veciana D, et al. Use of distinct anti‐hypertensive drugs and risk for COVID‐19 among hypertensive people: a population‐based cohort study in Southern Catalonia, Spain. J Clin Hypertens (Greenwich). 2020;22:1379–1388. doi: 10.1111/jch.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Cabezón Villalba G, Amat‐Santos IJ, Dueñas C, Lopez Otero D, Catala P, Aparisi A, López‐Pais J, Cacho Antonio CE, Candela J, Antúnez Muiños P, et al. Impact of the presence of heart disease, cardiovascular medications and cardiac events on outcome in COVID‐19. Cardiology J. 2021;28:360–368. doi: 10.5603/CJ.a2021.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Wander PL, Lowy E, Beste LA, Tulloch‐Palomino L, Korpak A, Peterson AC, Young BA, Boyko EJ. Risk factors for adverse outcomes among 35 879 veterans with and without diabetes after diagnosis with COVID‐19. BMJ Open Diabetes Res Care. 2021;9:e002252. doi: 10.1136/bmjdrc-2021-002252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Wang H‐Y, Peng S, Ye Z, Li P, Li Q, Shi X, Zeng R, Yao Y, He F, Li J, et al. Renin‐angiotensin system inhibitor is associated with the reduced risk of all‐cause mortality in COVID‐19 among patients with/without hypertension. Front Med. 2022;16:102–110. doi: 10.1007/s11684-021-0850-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Wang T, Tang R, Ruan H, Chen R, Zhang Z, Sang L, Su XI, Yi S, Ni Z, Hu YU, et al. Predictors of fatal outcomes among hospitalized COVID‐19 patients with pre‐existing hypertension in China. Clin Respir J. 2021;15:915–924. doi: 10.1111/crj.13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Xu J, Huang C, Fan G, Liu Z, Shang L, Zhou F, Wang Y, Yu J, Yang L, Xie KE, et al. Use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers in context of COVID‐19 outbreak: a retrospective analysis. Front Med. 2020;14:601–612. doi: 10.1007/s11684-020-0800-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Yahyavi A, Hemmati N, Derakhshan P, Banivaheb B, Karimi Behnagh A, Tofighi R, TehraniYazdi A, Kabir A. Angiotensin enzyme inhibitors and angiotensin receptor blockers as protective factors in COVID‐19 mortality: a retrospective cohort study. Intern Emerg Med. 2021;16:883–893. doi: 10.1007/s11739-020-02523-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Yan F, Huang F, Xu J, Yang P, Qin Y, Lv J, Zhang S, Ye LU, Gong M, Liu Z, et al. Antihypertensive drugs are associated with reduced fatal outcomes and improved clinical characteristics in elderly COVID‐19 patients. Cell Discov. 2020;6:77. doi: 10.1038/s41421-020-00221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Yan H, Valdes AM, Vijay A, Wang S, Liang L, Yang S, Wang H, Tan X, Du J, Jin S, et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID‐19: a large case‐control study. Clin Pharmacol Ther. 2020;108:1185–1194. doi: 10.1002/cpt.2047 [DOI] [PubMed] [Google Scholar]
- 228. Zhang P, Zhu L, Cai J, Lei F, Qin J‐J, Xie J, Liu Y‐M, Zhao Y‐C, Huang X, Lin L, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Zhang X‐J, Qin J‐J, Cheng XU, Shen L, Zhao Y‐C, Yuan Y, Lei F, Chen M‐M, Yang H, Bai L, et al. In‐hospital use of statins is associated with a reduced risk of mortality among individuals with COVID‐19. Cell Metab. 2020;32:176–187.e4. doi: 10.1016/j.cmet.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zhong Y, Zhao L, Wu G, Hu C, Wu C, Xu M, Dong H, Zhang Q, Wang G, Yu BO, et al. Impact of renin‐angiotensin system inhibitors use on mortality in severe COVID‐19 patients with hypertension: a retrospective observational study. J Int Med Res. 2020;48:300060520979151. doi: 10.1177/0300060520979151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Zhou F, Liu Y‐M, Xie J, Li H, Lei F, Yang H, Qin J‐J, Cai J, Zhang X‐J, Wu B, et al. Comparative impacts of ACE (angiotensin‐converting enzyme) inhibitors versus angiotensin II receptor blockers on the risk of COVID‐19 mortality. Hypertension. 2020;76:e15–e17. doi: 10.1161/HYPERTENSIONAHA.120.15622 [DOI] [PubMed] [Google Scholar]
- 232. Zhou X, Zhu J, Xu T. Clinical characteristics of coronavirus disease 2019 (COVID‐19) patients with hypertension on renin‐angiotensin system inhibitors. Clin Exp Hypertens. 2020;42:656–660. doi: 10.1080/10641963.2020.1764018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Amat‐Santos IJ, Santos‐Martinez S, López‐Otero D, Nombela‐Franco L, Gutiérrez‐Ibanes E, Del Valle R, Muñoz‐García E, Jiménez‐Diaz VA, Regueiro A, González‐Ferreiro R, et al. Ramipril in high‐risk patients with COVID‐19. J Am Coll Cardiol. 2020;76:268–276. doi: 10.1016/j.jacc.2020.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Bauer A, Schreinlechner M, Sappler N, Dolejsi T, Tilg H, Aulinger BA, Weiss G, Bellmann‐Weiler R, Adolf C, Wolf D, et al. Discontinuation versus continuation of renin‐angiotensin‐system inhibitors in COVID‐19 (ACEI‐COVID): a prospective, parallel group, randomised, controlled, open‐label trial. Lancet Respir Med. 2021;9:863–872. doi: 10.1016/S2213-2600(21)00214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Cohen JB, Hanff TC, William P, Sweitzer N, Rosado‐Santander NR, Medina C, Rodriguez‐Mori JE, Renna N, Chang TI, Corrales‐Medina V, et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID‐19: a prospective, randomised, open‐label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Duarte M, Pelorosso F, Nicolosi LN, Victoria Salgado M, Vetulli H, Aquieri A, Azzato F, Castro M, Coyle J, Davolos I, et al. Telmisartan for treatment of Covid‐19 patients: an open multicenter randomized clinical trial. EClinicalMedicine. 2021;37:100962. doi: 10.1016/j.eclinm.2021.100962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Geriak M, Haddad F, Kullar R, Greenwood KL, Habib M, Habib C, Willms D, Sakoulas G. Randomized prospective open label study shows no impact on clinical outcome of adding losartan to hospitalized COVID‐19 patients with mild hypoxemia. Infect Dis Ther. 2021;10:1323–1330. doi: 10.1007/s40121-021-00453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lopes RD, Macedo AVS, de Barros E Silva PGM, Moll‐Bernardes RJ, Dos Santos TM, Mazza L, Feldman A, D’Andréa Saba Arruda G, de Albuquerque DC, Camiletti AS, et al. Effect of discontinuing vs continuing angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID‐19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Najmeddin F, Solhjoo M, Ashraf H, Salehi M, Rasooli F, Ghoghaei M, Soleimani A, Bahreini M. Effects of renin‐angiotensin‐aldosterone inhibitors on early outcomes of hypertensive COVID‐19 patients: a randomized triple‐blind clinical trial. Am J Hypertens. 2021:hpab111. doi: 10.1093/ajh/hpab111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Puskarich MA, Cummins NW, Ingraham NE, Wacker DA, Reilkoff RA, Driver BE, Biros MH, Bellolio F, Chipman JG, Nelson AC, et al. A multi‐center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID‐19. EClinicalMedicine. 2021;37:100957. doi: 10.1016/j.eclinm.2021.100957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Renoux C, Azoulay L, Suissa S. Biases in evaluating the safety and effectiveness of drugs for the treatment of COVID‐19: designing real‐world evidence studies. Am J Epidemiol. 2021;190:1452–1456. doi: 10.1093/aje/kwab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, Montori V, Akl EA, Djulbegovic B, Falck‐Ytter Y, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2
Figure S1


