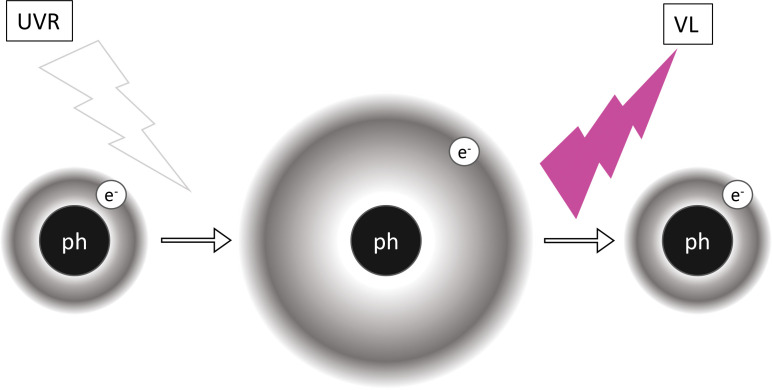
FIGURE 1.
Schematic of electronic transitions during fluorescence. When the relatively more energetic and shorter wavelengths of ultraviolet radiation (UVR) shine on a phosphor (ph), its electrons (e-) are moved to excited states. Since such excited states are unstable, electrons soon revert to their ground states. Energy decays in the system via vibrational relaxation and other means, and the remainder is emitted in relatively less energetic and longer wavelengths of visible light (VL).