FIG 1.
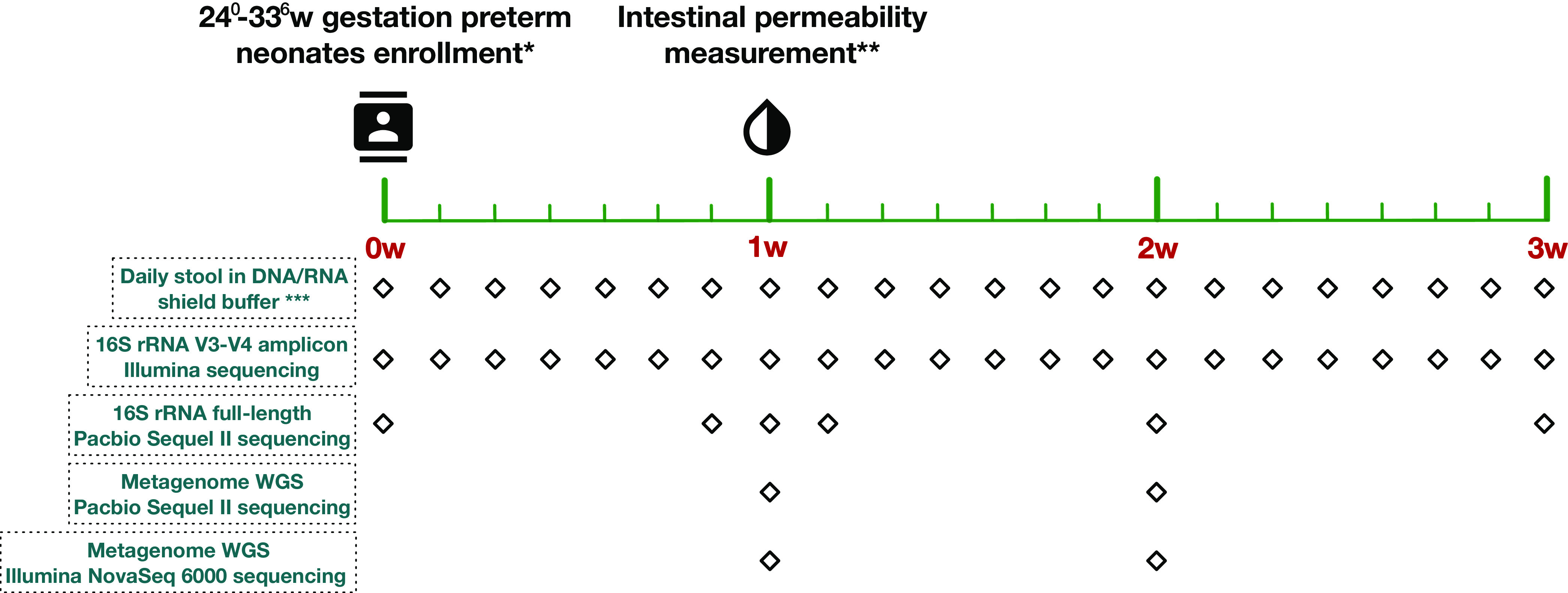
Study design. *, demographic, clinical, and nutritional information was collected for each enrolled preterm neonate. Inclusion criteria include 240 to 326 weeks and <4 days of age. Exclusion criteria include nonviable or planned withdrawal of care, severe asphyxia, chromosome abnormalities, cyanotic congenital heart disease, intestinal atresia or perforation, abdominal wall defects, significant GI dysfunction, and galactosemia or other forms of galactose intolerance. **, intestinal permeability was measured using the urine non-metabolized sugar probes lactulose and rhamnose at days 7 to 10 after birth. ***, stool specimens were collected daily at every stooling event, stored in storage buffer, and archived at −80°C. WGS, whole-genome sequencing.
