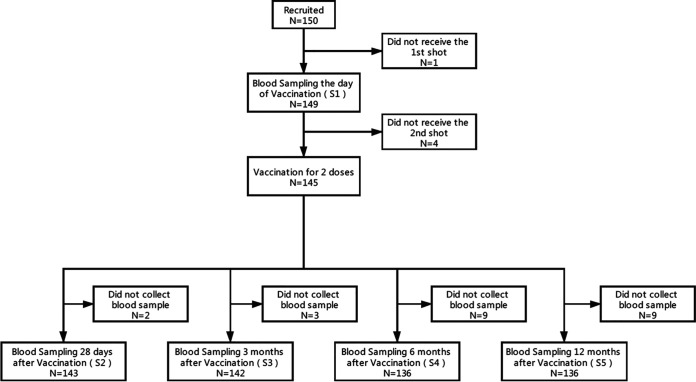
FIG 1.
Schedule of sample collection. A total of 150 participants aged 18 to 59 were enrolled in Beijing CDC, China. The participants were administered 3 μg CoronaVac intramuscularly following a 2-shot vaccine schedule, 14 days apart. Following that, the samples, including serum, plasma, and peripheral blood mononuclear cells, were collected on day 0 before vaccination (baseline) and at 1, 3, 6, and 12 months after the second shot. A total of 136 of participants completed the study through the 12-month endpoint.