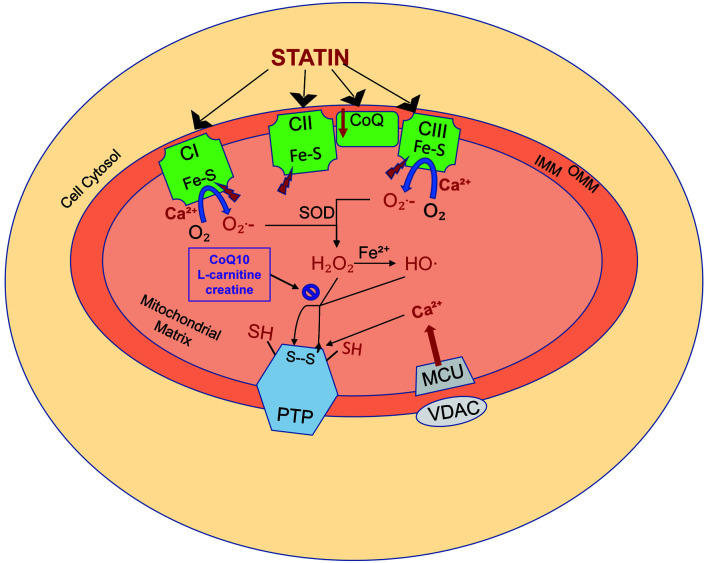
Fig. 1. Statin-induced mitochondrial oxidative stress.
Statins induce both mitochondrial oxidative stress and calcium-dependent permeability transition, which may lead to cell injury and death. (1) Statins diminish respiratory capacity at the level of complexes I (CI), II (CII), and III (CIII) of the respiratory chain, which in turn results in the increased generation of superoxide (O2−) generation. The iron sulfur centers (Fe-S) within the respiratory complexes are in turn inhibited, diminishing their resistance to calcium (Ca2+)-dependent mitochondrial permeability transition. (2) The mitochondrial antioxidant defense system uses superoxide dismutase to convert superoxide to hydrogen peroxide. Hydrogen peroxide is normally then metabolized by the mitochondrial antioxidant system, including coenzyme Q10 (CoQ10), L-carnitine, and creatine. With statin toxicity, a large quantity of hydrogen peroxide production results in the depletion of the antioxidant system. Remaining hydrogen peroxide induces membrane protein sulfhydryl-disulfide transition (SH, S–S), promoting permeability transition pore (PTP) opening. (3) Statins also impair cellular Ca2+homeostasis by increasing Ca2+release from the endoplasmic reticulum thereby increasing cytosolic Ca2+levels. Cytosolic Ca2+is taken up by a voltage-dependent anion-selective channel and mitochondrial calcium uniporter channels, leading to accumulation of Ca2+ in the mitochondrial matrix. The accumulated Ca2+ binds to the membrane, exposing specific buried thiols to the oxidants and impairing mitochondrial respiration, resulting in increased superoxide formation. The combination of increasing reactive oxygen species and mitochondrial Ca2+ load leads to PTP opening and subsequent cell death. OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane. Adapted from Busanello et al., 2017.48