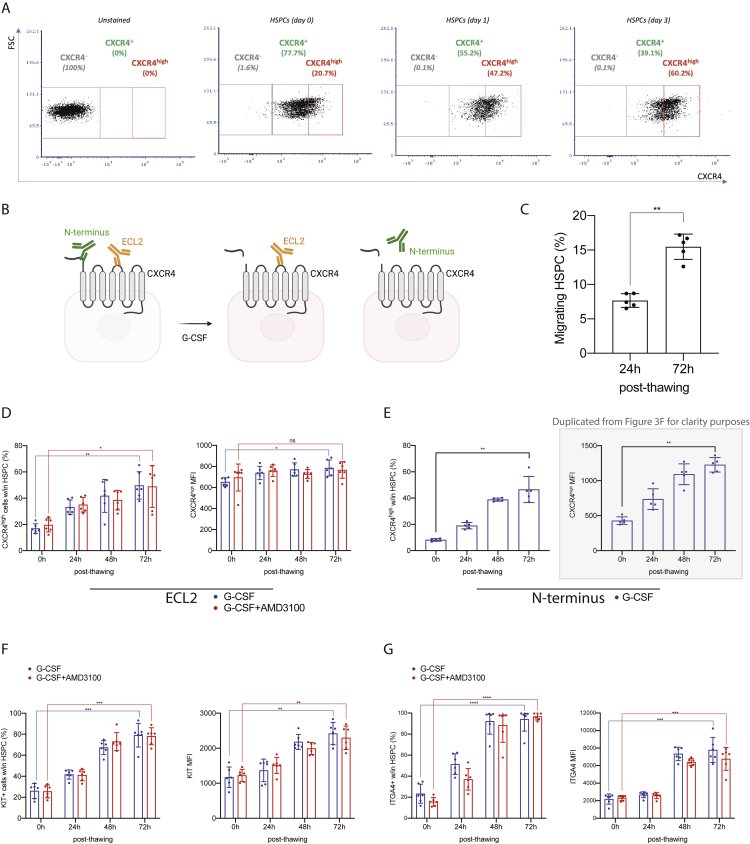
Figure S3.
M-HSCT allows an efficient donor to recipient exchange of HSPCs within the human niche of hematochimeric mice, related to Figure 3
(A) Representative plots showing the gating strategy used to characterize the CXCR4high population in the HSPC (CD34+CD133+CD90+) population.
(B) Scheme of CXCR4 cleavage following G-CSF mobilization and related antibodies localization.
(C) The percentage of migrating HSPCs, performed at 24 and 72 h post thawing, previously collected with G-CSF. A Kruskal-Wallis test was performed, followed by a post hoc analysis with Dunn’s test.
(D) The percentage of CXCR4high cells (left panel) and MFI (right panel) over time after thawing, stained with an antibody targeting the ECL2 epitope of CXCR4, on the HSPC population mobilized with G-CSF or G-CSF/AMD3100.
(E) The percentage of CXCR4high cells (left panel) and MFI (right panel) over time after thawing, stained with an antibody targeting the N terminus epitope of CXCR4, on the HSPC population mobilized with G-CSF.
(F) The percentage of KIT+ cells (left panel) and MFI (right panel) over time after thawing, on the HSPC population mobilized with G-CSF or G-CSF/AMD3100.
(G) The percentage of ITGA4+ cells (left panel) and MFI (right panel) over time after thawing, on the HSPC population mobilized with G-CSF or G-CSF/AMD3100. The longitudinal comparisons, performed by a mixed-effect model (REML), followed by a post hoc analysis with Dunnett’s test.
The results are mean ± SEM, with n ≧ 5, with 3 different donors. In all the analyses, p less than 0.05 were considered significant (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. “ns” means non-significance).