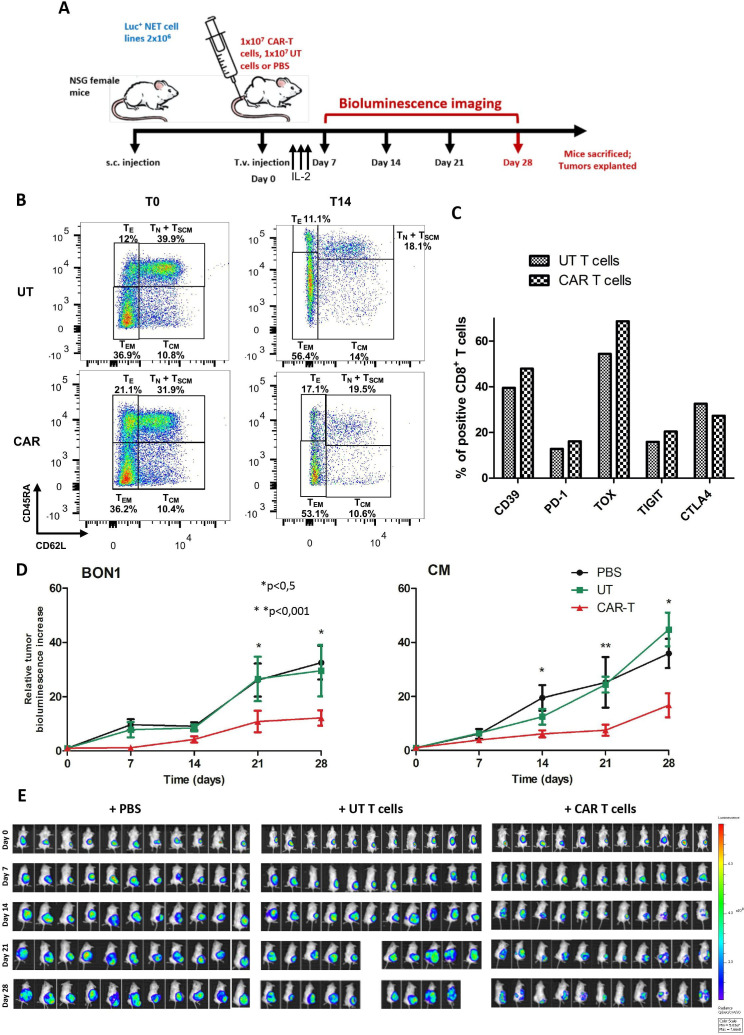
Figure 4.
Anti-SSTR CAR T cells effectively inhibit the growth of NET xenografts in vivo. (A) Experimental design of the adoptive cell transfer experiments in the NSG murine model. When the subcutaneous NET xenografts reached 1 mm3 in volume by caliper measurement, mice were randomized to receive PBS, UT T cells, or anti-SSTR CAR T cells by tail vein injection. Following treatment, mice received three daily intraperitoneal administration of recombinant human IL-2. The response to treatment was assessed once weekly by in vivo BLI, and tumor bioluminescence was normalized to baseline. After 4 weeks from treatment, mice were sacrificed and tumors, brain, pancreas and spleen were explanted. (B) Characterization of the T cell differentiation status of the adoptive products to be infused in mice. The flow plots of UT T cells and CAR T cells at the beginning (T0) and at the end of the ex vivo expansion phase (T14) are represented. (C) Characterization of the expression of exhaustion markers by UT T cells and CAR T cells before injection in mice. (D) Growth curves of BON1 and CM xenografts after treatment with anti-SSTR CAR T cells or UT T cells or PBS. Bioluminescence increase relative to baseline values is expressed as mean of 11 tumor-bearing mice±standard errors. *P<0.05; **p<0.01. (E) Bioluminescence images of NSG mice bearing CM xenografts from day 0 (i.v. infusion of PBS, UT T cells or CAR T cells) to day 28 (end of the experiment). Color scale for all images: min=5x107, max 7.7×108. CAR, chimeric antigen receptor; PBS: phosphate-buffered saline; SSTR, somatostatin receptor; UT, untransduced.