Table 1.
Reaction optimization
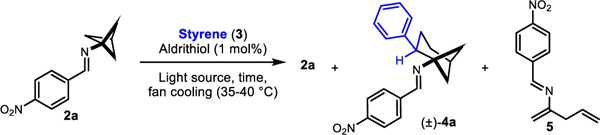
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | time (h) | 3 (eq) | Lamp (nm) | Solvent | 2ah % | 4ah % | 5h % |
| 1a,f | 3 | 7.5 | 390 | EtOAc | 6 | 15 | 59 |
| 2 a,f | 3 | 7.5 | 390 | CH3CN | 5 | 16 | 57 |
| 3 b,f | 3 | 7.5 | 390 | EtOAc | 7 | 29 | 40 |
| 4 a,f | 3 | 7.5 | 390 | CH3CN | 6 | 26 | 37 |
| 5 g | 3 | 7.5 | 390 | neat | 18 | 38 | 23 |
| 6 f | 3 | 15 | 390 | neat | 6 | 47 | 28 |
| 7 g | 3 | 7.5 | 370 | neat | 44 | 26 | 19 |
| 8 g | 3 | 7.5 | 427 | neat | 40 | 30 | 17 |
| 9 g | 3 | 7.5 | 440 | neat | 58 | 21 | 11 |
| 10 g | 3 | 7.5 | 456 | neat | 76 | 12 | 7 |
| 11 g | 3 | 7.5 | 525 | neat | 98 | 0 | 0 |
| 12 e,g | 3 | 7.5 | -- | neat | 99 | 0 | 0 |
| 13 c,g | 3 | 7.5 | 390 | neat | 20 | 33 | 20 |
| 14 d,g | 3 | 7.5 | 390 | neat | 22 | 37 | 22 |
| 15 c,g | 16 | 7.5 | 390 | neat | -- | 36 | 8 |
| 16 d,g | 16 | 7.5 | 390 | neat | -- | 36 | 9 |
| 17 g | 16 | 7.5 | 390 | neat | -- | 48 | 9 |
0.65 mL.
0.172 mL.
no Aldrithiol.
no freeze-pump-thaw.
60 °C and shielded from light.
0.2 mmol imine.
0.4 mmol imine.
yield based on Q1HNMR analysis with 1,3,5-trimethoxybenzene internal standard
