Figure 1.
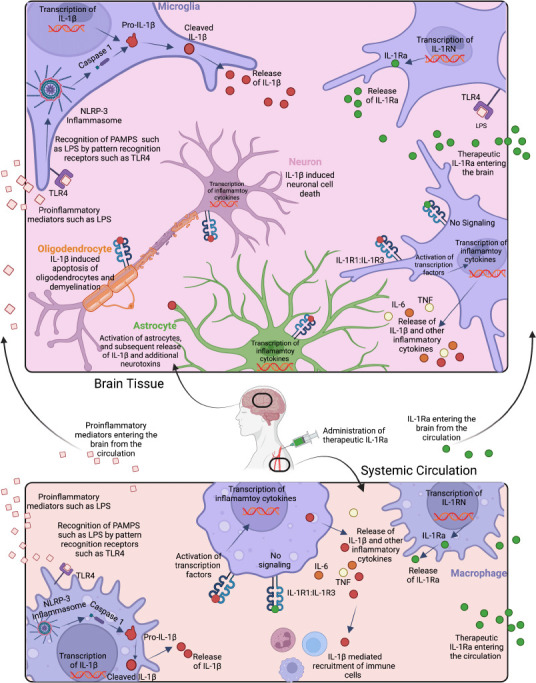
Innate immune stimulant-induced activation and signaling of IL-1β in brain (top) and circulation (bottom), and effects of endogenous and exogenous IL-1Ra.
Cerebral recognition of pathogen-associated molecular patterns (PAMPS) such as LPS by innate immune receptors, such as toll-like receptor 4 (TLR4) on microglia (purple), astrocytes (green), and other immune cells, leads to nuclear factor kappa B transcription of IL-1β. Consequently, the Pro-IL-1β protein is expressed intracellularly. The NOD-LRR-and pyrin domain-containing protein 3 (NLRP3) inflammasome complex cleaves pro-caspase 1 into caspase 1 which in turn cleaves pro-IL-1β into bioactive IL-1β, which is then secreted by the cell. Bioactive IL-1β reacts with the IL-1 heterodimeric receptor (IL-1R1:IL-1R3) on the surface of most cell types including other microglia, astrocytes, oligodendrocytes (orange), and neurons (pink), and leads to further nuclear factor kappa B regulated transcription of IL-1β and the subsequent release of bioactive IL-1β. Increased protein expression levels of IL-1β cause activation of astrocytes, as well as cell damage and apoptosis of oligodendrocytes and neurons. By contrast, the endogenous IL-1 receptor antagonist (IL-Ra) secreted by microglia, and administration of recombinant exogenous IL-1Ra, bind to the IL-1R1: IL-1R3 receptor complex and thereby block activation of transcription factors. In the circulation, LPS is detected by TLR4 on macrophages (purple), and the same IL-1β signaling cascade releases IL-1β into the circulation which recruits additional immune cells and can further damage the endothelium. The endogenous IL-1 receptor antagonist IL-1Ra released by macrophages and other immune cells, and recombinant exogenous IL-1Ra blocks IL-1 signaling. Created with BioRender.com.
