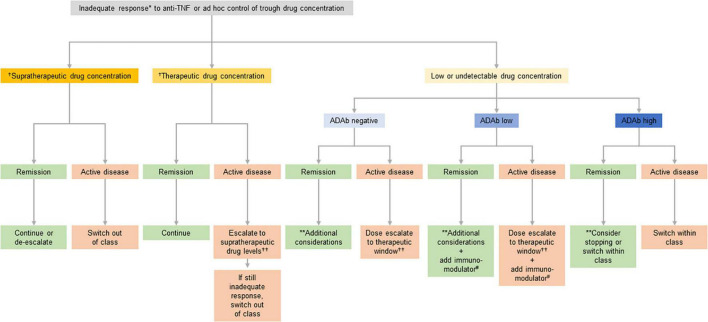
FIGURE 2.
Suggested clinical therapeutic drug monitoring (TDM)-based algorithm for optimizing anti-tumor necrosis factor (anti-TNF) therapy. *If disease activity is defined by symptoms confirm inflammatory activity and/or rule out potential non-inflammatory causes. Potential non-inflammatory causes of increased symptoms include fibrotic stricture, gastrointestinal infection, irritable bowel syndrome, bacterial overgrowth, bile salt diarrhea, colorectal cancer, and andamyloidosis. **This situation may be interpreted either as: (A) the patient being in remission despite not having any relevant anti-TNF activity (low/undetectable drug concentration) and thus it may be stopped; or (B) the patient is in the first step toward a potential relapse according to the multi-step hypothesis suggesting that the first step toward a relapse is a decline in drug concentration, the second step an increase in subclinical inflammation, and the final step a clinical relapse, and thus the drug concentration should be brought back to the therapeutic window. Deciding on which of the two is most likely involves taking several aspects into account including the patient’s disease history, comorbidities, and concomitant medications. †See Table 2 for suggested supratherapeutic and therapeutic drug concentrations. ††Both increase in dose (at standard doses) and increase in frequency are appropriate but maintaining the dose interval saves on nurse/infusion-related resources. #Immunomodulator defined as azathioprine or methotrexate.