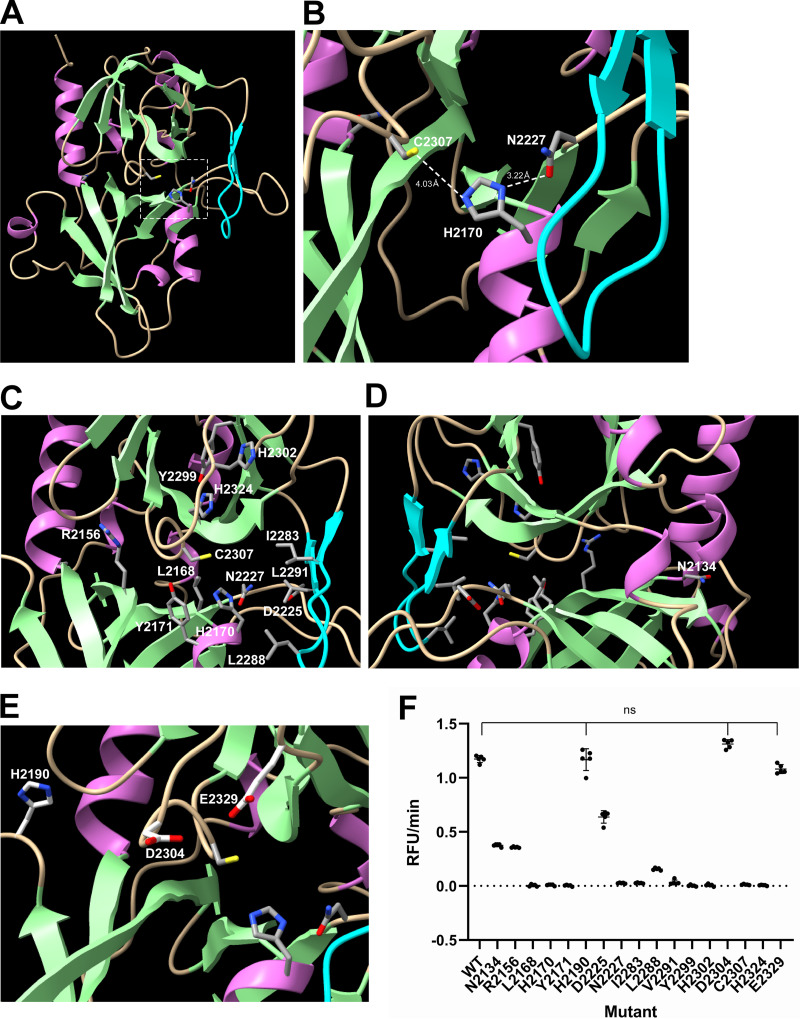
FIG 4.
Structure–function analysis of the deformed wing virus (DWV) 3C protease (3Cpro). (A) Structure of DWV 3Cpro predicted by AlphaFold2. The helix, strand, and coil structures are indicated by orchid, pale green, and wheat colors, respectively. β-ribbon is colored by cyan. The catalytic Cys-His-Asn triad is indicated by a square with a white dotted line. (B) Close-up view of the catalytic C2307-H2170-N2227 triad with distance information. (C) Positions of amino acid residues critical for the protease activity. (D) The position of N2134 that was well conserved between 3Cpros of Iflaviruses is shown by rotating the image (C) by 180°. (E) The positions of H2190, D2304, and E2329, which are not essential for the protease activity, are shown together with the catalytic C2307-H2170-N2227 triad. (F) Protease activities of wild type (WT) 3Cpro and alanine substituted mutants. Compared to WT, all mutants showed decreased activities, except H2190A, D2304A, and E2329A (ns), as indicated by the Dunnett test (one-tailed, n = 5).