FIG 6.
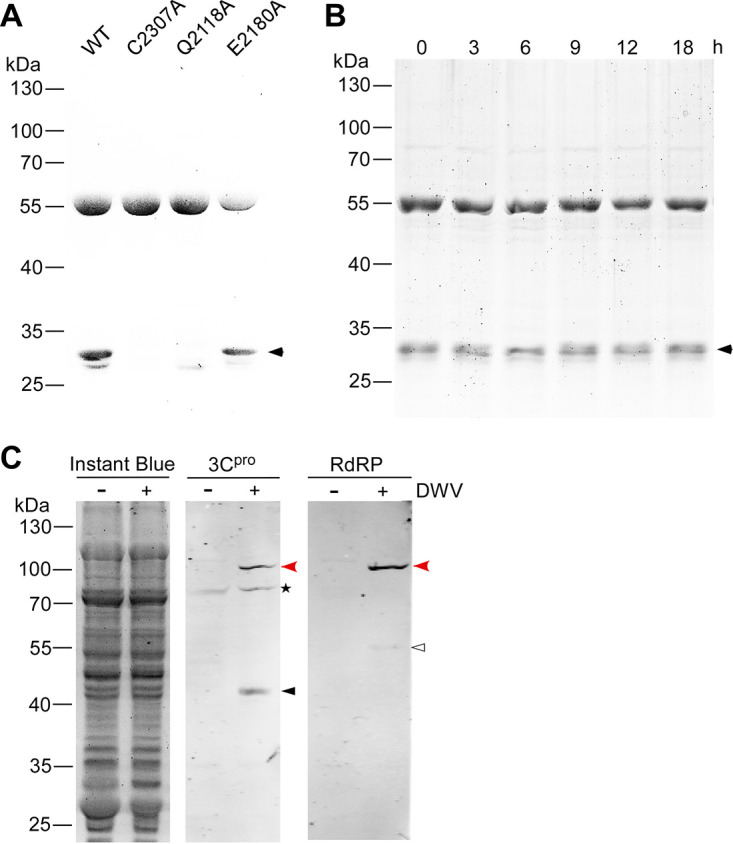
Self-cleavage of the deformed wing virus (DWV) 3C protease (3Cpro) and its synthesis in the virus-infected honey bee cells. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of purified wild type (WT) and three mutant (C2307A, Q2118A, and E2180A) glutathione S-transferase (GST)-DWV 3Cpro proteins. The 30 kDa band co-purified with 56 kDa GST-DWV 3Cpro is indicated by a black arrowhead. Molecular weights (kDa) of the protein marker are at the left. (B) SDS-PAGE of purified GST-DWV 3Cpro incubated at 33°C for the indicated time. (C) Western blotting of lysates of the control (−) or DWV-infected (+) honey bee pupal head cells by anti-3Cpro (3Cpro) or anti-RdRP (RdRP) antibody. The replicate gel was stained with Instant Blue to show that equal amount of protein was applied in each lane. Mature 42 kDa 3Cpro and 55 kDa RdRP are indicated by black and white arrowheads, respectively. The 97 kDa precursor of 3Cpro and RdRP (red arrowheads) was detected by both anti-3Cpro and anti-RdRP antibodies. The above-mentioned three bands were specifically present in DWV-infected cells. Asterisk represents the nonspecific band detected by the anti-3Cpro antibody.
