Abstract
Introduction
Maternal SARS-CoV-2 infection during pregnancy is associated with adverse pregnancy outcomes and can have effects on the placenta, even in the absence of severe disease or vertical transmission to the fetus. This study aimed to evaluate histopathologic and molecular effects in the placenta after SARS-CoV-2 infection during pregnancy.
Methods
We performed a study of 45 pregnant participants from the Generation C prospective cohort study at the Mount Sinai Health System in New York City. We compared histologic features and the expression of 48 immune and trophoblast genes in placentas delivered from 15 SARS-CoV-2 IgG antibody positive and 30 IgG SARS-CoV-2 antibody negative mothers. Statistical analyses were performed using Fisher's exact tests, Spearman correlations and linear regression models.
Results
The median gestational age at the time of SARS-CoV-2 IgG serology test was 35 weeks. Two of the IgG positive participants also had a positive RT-PCR nasal swab at delivery. 82.2% of the infants were delivered at term (≥37 weeks), and gestational age at delivery did not differ between the SARS-CoV-2 antibody positive and negative groups. No significant differences were detected between the groups in placental histopathology features. Differential expression analyses revealed decreased expression of two trophoblast genes (PSG3 and CGB3) and increased expression of three immune genes (CXCL10, TLR3 and DDX58) in placentas delivered from SARS-CoV-2 IgG positive participants.
Discussion
SARS-CoV-2 infection during pregnancy is associated with gene expression changes of immune and trophoblast genes in the placenta at birth which could potentially contribute to long-term health effects in the offspring.
Keywords: SARS-CoV-2, Placenta, Gene expression, NanoString
1. Introduction
The current pandemic of coronavirus disease 2019 (Covid-19) is caused by infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of January 24, 2022 approximately 352 million infections and 5.6 million deaths have been reported worldwide [1]. The clinical manifestations of SARS-CoV-2 infection vary widely by age group and presence of comorbid conditions, ranging from asymptomatic infection to respiratory failure, multisystem organ failure, and death in critically ill patients [2,3]. Although comprehensive knowledge of the factors involved in severe Covid-19 is still needed, dysregulation of the host inflammatory and immune responses [4,5] and thrombosis [6] have been implicated in Covid-19 induced tissue damage.
During pregnancy, the maternal immune system undergoes a series of dynamic changes aimed to promote tolerance of the fetus, that can also influence responses to pathogens, including viruses [7]. Recent reports show that pregnant individuals with severe SARS-CoV-2 infection are at higher risk of intensive care unit admission, mechanical ventilation, extracorporeal membrane oxygenation, and mortality compared to non-pregnant individuals [8,9]. Other studies show that active SARS-CoV-2 infection at delivery (mainly confirmed through a PCR positive test) is associated with obstetric and neonatal complications including increased risk of preterm birth, stillbirth, miscarriage, preeclampsia, emergency cesarean section and higher neonatal morbidity [8,[10], [11], [12], [13], [14], [15], [16], [17], [18]]. However, in other reports, including ours from New York City and a Denmark study, SARS-CoV-2 IgG seropositivity without RT-PCR positivity at delivery was not associated with adverse pregnancy outcomes [19,20].
The main cell entry pathway of the SARS-CoV-2 virus is dependent on the ACE2 receptor and aided by the TMPRSS2 proteases of the host cells [21].Early in pregnancy, ACE2 and TMPRSS2 are expressed in placental cytotrophoblast and syncytiotrophoblast cells; however, the expression of these proteins is low in term placentas [[22], [23], [24]]. Recent data suggest that multiple placental cell types are susceptible to infection in explants and immortalized cells after exposure to SARS-CoV-2, and infection susceptibility is related to the levels of ACE2 expression [[25], [26], [27]]. Yet, existing data show that vertical transmission of SARS-CoV-2 is rare; only a few reports have documented presence of the virus in the fetal compartments of the placenta or in newborns [[28], [29], [30]]. Moreover, most of the histopathology studies suggest that placental infection with SARS-CoV-2 is also rare [[31], [32], [33]] and can exist in the absence of vertical transmission [34,35]. Additionally, the criteria to diagnose SARS-CoV-2 placental infection have been inconsistent across studies [34,[36], [37], [38], [39], [40]]. Nonspecific placental histopathologic lesions have also been associated with maternal SARS-CoV-2 infection; a recent meta-analysis reports increases in risk of fetal vascular malperfusion, acute and chronic proinflammatory lesions, increased perivillous fibrin, and intervillous thrombosis [41]. These lesions could result from localized placental SARS-CoV-2 infection and/or inflammatory responses to the systemic maternal infection. However, most of the available reports evaluated placental effects of acute SARS-CoV-2 infections mainly from mothers infected at delivery (positive nasopharyngeal PCR test). To date, only few studies have reported changes in expression of immune and inflammatory genes in the placenta, also in cases of acute SARS-CoV-2 infection [[42], [43], [44]]. Understanding the impact of maternal SARS CoV-2 infection on the placenta during pregnancy, including among participants without active infection at delivery, is vital because these placental changes can lead to adverse pregnancy outcomes and long-term effects on the health of newborns [45]. The aim of this work was to evaluate histopathologic and gene expression changes in placentas delivered from SARS-CoV-2 IgG positive compared to those from SARS-CoV-2 IgG negative pregnant individuals.
2. Methods
2.1. Study population
The Generation C study is a prospective pregnancy cohort study that aims to examine the impact of SARS-CoV-2 infection during pregnancy on obstetric and neonatal outcomes. Pregnant individuals were recruited at Mount Sinai Hospital (MSH) and Mount Sinai West (MSW) in New York City (NYC) starting April 20, 2020, and recruitment is ongoing [19]. The first COVID-19 case in NYC was officially confirmed on March 1, 2020. Maternal blood samples are collected at multiple time points as part of routine clinical care. Electronic medical record (EMR) review and serological SARS-CoV-2 IgG tests are used to confirm past SARS-CoV-2 infection. Serological testing for IgG antibodies against the SARS-CoV-2 spike (S) protein (anti-S IgG) was performed using an enzyme-linked immunosorbent assay (ELISA) developed at the Icahn School of Medicine at Mount Sinai [46]. Placental samples are collected after delivery by the Mount Sinai Biorepository and Pathology Core. All participants provided written informed consent per the institutional review board (IRB)-approved study protocol (IRB at the Icahn School of Medicine at Mount Sinai, protocol IRB-20-03352, April 15, 2020).
For this analysis, we examined a subset of Generation C participants with and without evidence of past SARS-CoV-2 infection with available placenta tissue blocks collected for medical pathology examination and consent to donate placental tissue; 15 participants were SARS-CoV-2 IgG positive and 30 were SARS-CoV-2 IgG negative. EMR review was conducted to obtain clinical and sociodemographic characteristics of mothers and infants. Participants in this analysis gave birth between May and September of 2020. In NYC, widespread community transmission of SARS-CoV-2 in NYC began in March 2020, thus an IgG positive tests in a pregnant participant who gave birth before or in late September suggests that they were infected at some point during pregnancy, although we cannot confirm the exact timing of the infection. Additionally, all 45 pregnant participants delivered before the first COVID-19 vaccine received Emergency Use Authorization by the U.S. FDA in December 2020.
2.2. Placenta histopathology
After fixation, placentas were processed according to standard protocols including comprehensive gross tissue and histopathologic examination of the umbilical cord, chorionic membranes, and placental villi. Histopathologic review was performed according to the Amsterdam Placental Workshop Group Consensus Statement guidelines [47]. All the placentas in the study were reviewed for medical pathology and findings were recorded in the pathology report and in the EMR.
2.3. Targeted placental gene expression profiling
RNA was extracted from formaldehyde-fixed paraffin embedded (FFPE) tissue blocks using the Maxwell® 16 LEV RNA FFPE Purification Kit (Promega, Madison, WI). RNA concentration was determined using the Nanodrop (Thermo Fisher Scientific, MA). Gene expression was profiled with a custom designed NanoString codeset (NanoString, Seattle, WA) panel with 50 probes including genes involved in the inflammatory and/or immune response (n = 25), stress response (n = 8), cell-type markers (n = 7), SARS-CoV-2 host response (n = 6), viral SARS-CoV-2 genes (nucleocapsid and envelope proteins) and two housekeeping genes (RPL19, RPLP0) (Supplementary Table 1). RNA (100 ng) was hybridized overnight to reporter and capture probes at 65 °C. Next, unbound probes were removed, and purified complexes were aligned and immobilized on four NanoString cartridges using the nCounter Prep station. Cartridges were scanned for gene counts detection in the nCounter Digital Analyzer. All laboratory protocols we performed following manufacturer's instructions. Raw gene expression counts were imported from RCC files using the NanoStringNorm R package (1.2.1.1) [48]. To normalize CodeCount technical variation we used the geometric mean. Background expression levels were calculated based on the mean ± 2SD of negative control probes. Values below the background limit of detection (LOD) for each sample (mean ± 2SD of negative control probes) were replaced with LOD/√2. We used the geometric mean of the housekeeping genes to normalize for sample RNA sample content. After normalization, counts were log2 transformed for statistical analyses. Samples with less than 50% of probes above background were excluded (n = 1, SARS-CoV-2 IgG negative), and probes with counts below the background level in more than 50% of the samples in each study group were removed (n = 9). We used the placental-cell gene markers in the panel to calculate cell-type scores as the average of the log2 normalized expression of each of the cell type gene marker (PEG10 and PEG3 for cytotrophoblasts, CGB3 and PSG3 for syncytiotrophoblasts, CD68 and CD163 for macrophages and PECAM1 for endothelial cells).
2.4. Statistical analyses
We used summary statistics including median, range, or frequency tables to evaluate the distribution of continuous and categorical variables. We performed bivariate analyses using Fisher exact tests and Wilcoxon signed-rank or Kruskal–Wallis tests as appropriate to evaluate differences in clinical, sociodemographic, and histopathology variables between the SARS-CoV-2 IgG positive versus negative groups. We used principal components analyses to evaluate possible effects of technical (e.g., NanoString cartridge) or biological (e.g., infant sex) covariates in placental gene expression. Differential gene expression analysis by SARS-CoV-2 IgG status was performed using the Limma R package [49] that uses an empirical Bayes method to fit linear models with moderated standard errors for each gene (continuous outcome variable) and the study group (IgG positive versus negative) as the predictor variable. We considered as possible confounders covariates that could influence placenta gene expression including infant sex, birthweight, gestational age at birth, maternal age, maternal pre-pregnancy BMI and gestational age at SARS-CoV-2 IgG antibody test. The final linear models were adjusted for covariates (infant sex, gestational age at birth, birthweight, maternal age, and pre-pregnancy BMI) and cell-type proxy scores. Sensitivity analyses were performed excluding two participants with acute infections at delivery to evaluate the impact of SARS-CoV-2 PCR positivity at delivery. Statistical significance was set at p ≤ 0.05. Analyses were performed in R statistical computing software version 4.1.0 [50].
3. Results
3.1. Demographic, clinical and placenta histopathologic characteristics
Table 1 displays the characteristics of the Generation C participants included in these analyses (n = 45) stratified by study groups: SARS-CoV-2 IgG negative (n = 30) and SARS-CoV-2 IgG positive (n = 15). The median gestational age of IgG serology testing was 35 weeks with an interquartile range (IQR) between 17.4 and 40.9 weeks. Two of the IgG positive participants were also SARS-CoV-2 PCR positive (nasopharyngeal swab) at the time of the labor and delivery admission. Like in the larger Generation C cohort [19], IgG seropositive participants had higher pre-pregnancy BMI (p = 0.05) and were more often Hispanic, or non-Hispanic Black compared to IgG seronegative participants. In contrast, we did not observe differences in other maternal characteristics including age, parity, tobacco use, medical conditions, or obstetric conditions.
Table 1.
Maternal characteristics stratified by SARS-CoV-2 IgG status.
| Total n = 45 | SARS-CoV-2 IgG negative n = 30 | SARS-CoV-2 IgG positive n = 15 | p-value* | |
|---|---|---|---|---|
| Maternal age | 34 [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] | 35 [[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]] | 33 [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] | 0.21 |
| Pre-pregnancy BMI | 25.4 [17.3–59.7] | 24.9 [17.3–59.7] | 28.3 [21.2–39.6] | 0.05 |
| Gestational age (wks) at IgG serology | 34.9 [17.4–40.9] | 35.4 [17.4–40.9] | 34.1 [21–36.9] | 0.24 |
| SARS-CoV-2 status at delivery | ||||
| IgG-negative/PCR-negative | 30 (66.7%) | 30 (100%) | 0 (0%) | |
| IgG-positive/PCR-negative | 13 (28.9%) | 0 (0%) | 13 (86.7%) | |
| IgG-positive/PCR-positive | 2 (4.4%) | 0 (0%) | 2 (13.3%) | |
| Maternal Race/Ethnicity | 0.10 | |||
| Asian | 5 (11.1%) | 4 (13.3%) | 1 (6.7%) | |
| Black, non-Hispanic | 6 (13.3%) | 2 (6.7%) | 4 (26.7%) | |
| Hispanic | 3 (6.7%) | 1 (3.3%) | 2 (13.3%) | |
| Other | 9 (20%) | 5 (16.7%) | 4 (26.7%) | |
| White, non-Hispanic | 21 (46.7%) | 17 (56.7%) | 4 (26.7%) | |
| Unknown | 1 (2.2%) | 1 (3.3%) | 0 (0%) | |
| Parity | 1 | |||
| multiparous | 20 (44.4%) | 13 (43.3%) | 7 (46.7%) | |
| nulliparous | 25 (55.6%) | 17 (56.7%) | 8 (53.3%) | |
| Tobacco | 0.28 | |||
| Never | 41 (91.1%) | 26 (86.7%) | 15 (100%) | |
| Quit | 4 (8.9%) | 4 (13.3%) | 0 (0%) | |
| Gestational diabetes | 1.00 | |||
| No | 35 (77.8%) | 23 (76.7%) | 12 (80%) | |
| Yes | 10 (22.2%) | 7 (23.3%) | 3 (20%) | |
| Gestational hypertension | 0.65 | |||
| No | 39 (86.7%) | 25 (83.3%) | 14 (93.3%) | |
| Yes | 6 (13.3%) | 5 (16.7%) | 1 (6.7%) | |
| Chronic hypertension | 0.59 | |||
| No | 41 (91.1%) | 28 (93.3%) | 13 (86.7%) | |
| Yes | 4 (8.9%) | 2 (6.7%) | 2 (13.3%) | |
| Asthma | 0.70 | |||
| No | 37 (82.2%) | 24 (80%) | 13 (86.7%) | |
| Yes | 8 (17.8%) | 6 (20%) | 2 (13.3%) | |
| Preeclampsia | 0.28 | |||
| No | 41 (91.1%) | 26 (86.7%) | 15 (100%) | |
| Yes | 4 (8.9%) | 4 (13.3%) | 0 (0%) | |
| HELLP | 1.00 | |||
| No | 44 (97.8%) | 29 (96.7%) | 15 (100%) | |
| Yes | 1 (2.2%) | 1 (3.3%) | 0 (0%) | |
Categorical variables are frequencies and percentages (%). Continuous variables summarized with median [range]. * Wilcoxon test for continuous variables, fisher test for categorical variables. HELLP: Hemolysis, Elevated Liver enzymes and Low Platelets syndrome.
The characteristics of the newborns are shown in Table 2 . All were live births, 82.2% (n = 37) delivered at term, and 17.8 delivered preterm. We did not detect differences in gestational age between study groups; median gestational age at delivery was 38.9 and 39 weeks for newborns born to IgG-negative and IgG-positive participants, respectively (p = 0.87). The distribution of newborn sex was slightly different; 66.7% of newborns in the seropositive group were female and 33.3% were male, while in the seronegative group 33.3% were female and 66.7% were male (p = 0.06). No differences were noted in birthweight, delivery mode, intrauterine growth restriction, APGAR scores, or NICU admission rates.
Table 2.
Newborn characteristics stratified by maternal SARS-CoV-2 IgG status.
| Total n = 45 | SARS-CoV-2 IgG negative n = 30 | SARS-CoV-2 IgG positive n = 15 | p-valuea | |
|---|---|---|---|---|
| Birth weight (Kg) | 3.38 [2.2–3.93] | 3.23 [2.32–3.93] | 3.44 [2.19–3.79] | 0.90 |
| Gestational age (wks) at birth | 38.9 [34–41.6] | 38.9 [35.4–40.9] | 39 [34–41.6] | 0.87 |
| APGAR at 1 min | 9 [[4], [5], [6], [7], [8], [9]] | 8 [[4], [5], [6], [7], [8], [9]] | 9 [[7], [8], [9]] | 0.31 |
| APGAR at 5 min | 9 [8,9] | 9 [8,9] | 9 [9–9] | 0.14 |
| Infant sex | 0.06 | |||
| Female | 20 (44.4%) | 10 (33.3%) | 10 (66.7%) | |
| Male | 25 (55.6%) | 20 (66.7%) | 5 (33.3%) | |
| Birth weight group | 0.49 | |||
| AGA | 37 (82.2%) | 23 (76.7%) | 14 (93.3%) | |
| LGA | 4 (8.9%) | 3 (10%) | 1 (6.7%) | |
| SGA | 4 (8.9%) | 4 (13.3%) | 0 (0%) | |
| Delivery mode | 0.75 | |||
| C-Section | 18 (40%) | 13 (43.3%) | 5 (33.3%) | |
| Vaginal | 27 (60%) | 17 (56.7%) | 10 (66.7%) | |
| IUGR | 0.54 | |||
| No | 42 (93.3%) | 27 (90%) | 15 (100%) | |
| Yes | 3 (6.7%) | 3 (10%) | 0 (0%) | |
| Preterm birth | 1 | |||
| No | 37 (82.2%) | 25 (83.3%) | 12 (80%) | |
| Yes | 8 (17.8%) | 5 (16.7%) | 3 (20%) | |
| NICU admission | 0.70 | |||
| No | 36 (80%) | 23 (76.7%) | 13 (86.7%) | |
| Yes | 9 (20%) | 7 (23.3%) | 2 (13.3%) | |
Continuous variables summarized with median [range].
Categorical variables are frequencies and percentages (%).
Wilcoxon test for continuous variables, Fisher's test for categorical variables.
The pathology examination showed that placental weight was comparable between IgG seropositive and seronegative participants. Similarly, study groups were not different in other histopathology findings including chronic villitis, deciduitis, acute chorioamnionitis, intervillitis, intervillous thrombosis, fetal vascular thrombosis, decidual arteriopathy, fibrin presence, or chorangiosis (Table 3 ).
Table 3.
Histopathology characteristics stratified by maternal SARS-CoV-2 IgG status.
| Total n = 45 | SARS-CoV-2 IgG negative n = 30 | SARS-CoV-2 IgG positive n = 15 | p-valuea | |
|---|---|---|---|---|
| Placental weight (g) | 501 [304–644] | 476 [304–644] | 504 [317–568] | 0.92 |
| Chronic villitis | 2 (4.4%) | 1 (3.3%) | 1 (6.7%) | 1 |
| Deciduitis | 2 (4.4%) | 2 (6.7%) | 0 (0%) | 0.55 |
| Acute chorioamnionitis | 10 (22.2%) | 6 (20%) | 4 (26.7%) | 0.71 |
| Intervillitis | 1 (2.2%) | 0 (0%) | 1 (6.7%) | 0.33 |
| Intervillous thrombosis | 2 (4.4%) | 2 (6.7%) | 0 (0%) | 0.55 |
| Fetal vascular thrombosis | 1 (2.2%) | 1 (3.3%) | 0 (0%) | 1.00 |
| Decidual arteriopathy | 5 (11.1%) | 5 (16.7%) | 0 (0%) | 0.15 |
| Fibrin | 32 (71.1%) | 22 (73.3%) | 10 (66.7%) | 0.73 |
| Chorangiosis | 2 (4.4%) | 2 (6.7%) | 0 (0%) | 0.55 |
| Any inflammatory lesionb | 14 (31.3%) | 7 (23.3%) | 7 (46.7%) | 0.17 |
Continuous variables summarized with median [range]. Categorical variables are frequencies and percentages (%).
Wilcoxon test for continuous variables, Fisher's exact test for categorical variables.
Combined variable including acute chorioamnionitis, chronic villitis, intervillitis and deciduitis.
3.2. Placenta gene expression analysis
After preprocessing, quality control, the gene expression dataset consisted of 44 samples (IgG negative n = 29; IgG positive n = 15) and 48 genes. Nine genes were consistently below the background level in both study groups and were excluded from differential expression analyses. These included the nucleocapsid and the envelope viral SARS-CoV-2 genes, SARS-CoV-2 cell-entry genes (TMPRSS2, ACE2), and genes involved in the immune and stress response (IL17A, IL23A, IFNL3, IFNA1 and OPRM1). Summary statistics for the expression of the 39 detected genes are shown in Supplementary Table 2. We used principal components analyses to identify effects of biologic and technical covariates in the overall placental gene expression patterns, which did not reveal obvious clustering by SARS-CoV-2 IgG serology or nasal swab PCR status at delivery (Supplementary Fig. 1).
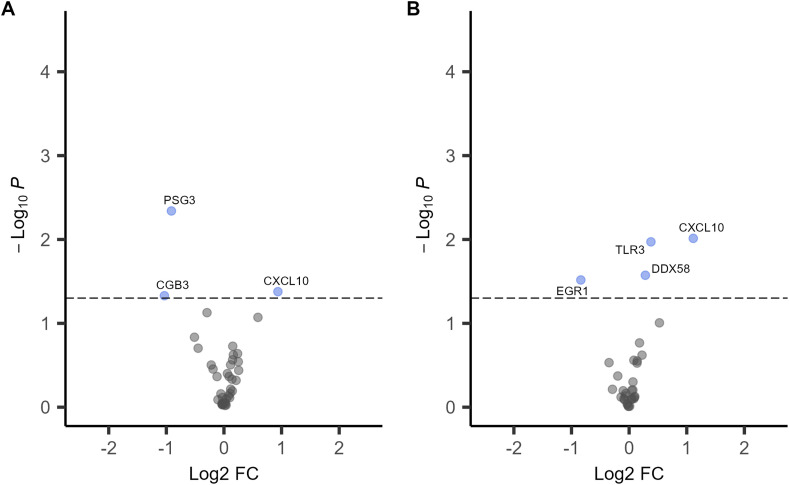
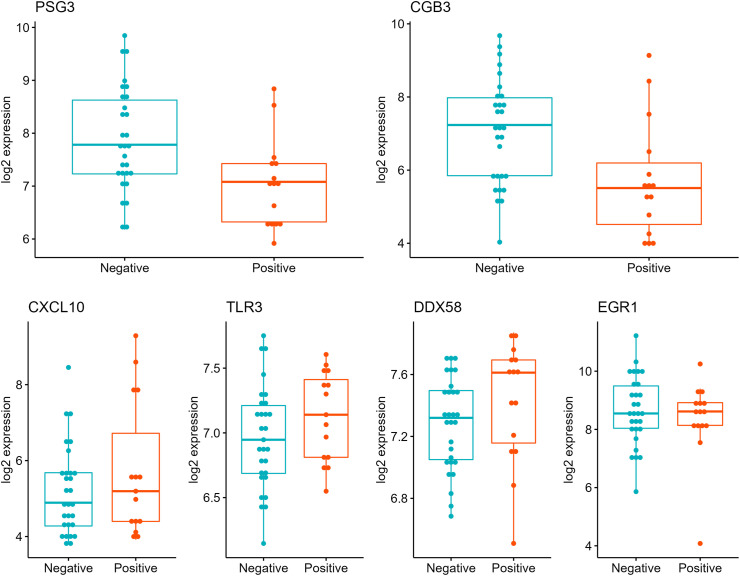
Next, we performed differential expression analyses by SARS-CoV-2 IgG status adjusted for covariates and cell-type proxies (Fig. 1 ). In the analyses adjusted for covariates only, three genes were significantly associated with SARS-CoV-2 IgG antibody status (Fig. 1A, Supplementary Table 3). The trophoblast cell-markers PSG3 and CGB3 were downregulated (log2 fold-change [log2FC] = - 0.9, p = 0.005 and log2FC = − 1.03, p = 0.05, respectively). PSG3 and CGB3 placental expression levels were highly correlated (rho = 0.6, p = 6.1 × 10−5). The chemokine CXCL10 was over-expressed (log2FC = 0.94, p = 0.04) in the IgG positive group compared to the IgG negative group. Since placental tissues are heterogenous mixtures of cells, we repeated the differential expression analyses adjusting for covariates and expression cell-type proxies including the average expression PSG3 and CGB3 as syncytiotrophoblast markers. In this analysis, SARS-CoV-2 IgG positivity was associated with increased expression of three genes CXCL10, TLR3 and DDX58 and downregulation of EGR1 (log2FC = − 0.84, p = 0.03) (Fig. 1B, Supplementary Table 4). The log2 normalized placenta expression values for these genes stratified by SARS-CoV-2 IgG positive and negative groups are depicted in Fig. 2 . Sensitivity analyses excluding samples from the two participants who were SARS-CoV-2 IgG positive and PCR positive at delivery showed similar results (Supplemental Fig. 2).
Fig. 1.
Volcano plots of differential gene expression analyses in placentas from SARS-CoV-2 IgG positive (n = 15) versus IgG negative (n = 29) participants. A. Linear models adjusted for infant sex, birthweight, gestational age at birth, maternal age and pre-pregnancy BMI. B. Linear models adjusted for covariates and cell-type gene expression proxies. The x-axis is the log2 fold change (FC) and y-axis is the –log10 P-value, the highlighted in blue are genes with p < 0.05. The horizontal dashed line corresponds to p = 0.05.
Fig. 2.
Box plots of gene expression in placentas delivered by SARS-CoV-2 IgG positive versus IgG negative participants. Top panel. PSG3 and CGB3 trophoblast genes. Bottom panel CXCL10, TLR3, DDX58 and EGR1 genes. The y-axis is the log2 fold change (FC) and x-axis is SARS-CoV-2 IgG antibody group.
4. Discussion
The effects of SARS-CoV-2 infection on the placenta are not yet well-characterized, particularly in cases of infection during pregnancy without active infection at delivery (i.e., SARS-CoV-2 IgG positive and negative PCR at the delivery admission). In this report, we investigated histopathology and molecular gene expression changes in placentas from 15 mother-infant pairs exposed to SARS-CoV-2 during pregnancy and 30 unexposed controls, part of the Generation C study in NYC. We report differences in the expression of trophoblast specific and immune genes in placentas between IgG positive and negative mothers.
All the placentas in this study underwent medical pathology review, and we found a range of histopathologic findings. However, none of the findings differed significantly with respect to SARS-CoV-2 IgG serology status, which is consistent with some studies on Covid-19 and placenta pathology [39,51]. A recent systematic review and pooled analysis of case-control reports found increased risk of fetal vascular malperfusion, chronic inflammatory pathology, perivillous fibrin, and intervillous thrombosis [41], yet most of the reviewed cases in that analyses were RT-PCR positive at delivery. Our results are inconsistent with these findings, possibly due to limited sample size or because our study population consisted mostly of individuals who were infected with the virus during pregnancy without active infection at delivery.
We detected significant associations between plasma SARS-CoV-2 IgG positivity and lower placental expression of the PSG3 and CGB3. These placenta-specific genes locate to 19q13 and are highly expressed by trophoblast cells. PSG3 is part of the family of human pregnancy-specific glycoproteins (PSG) that are released to the maternal circulation during pregnancy and are reported to have immunoregulatory and angiogenic functions [52]. CGB3 encodes the beta 3 subunit of the chorionic gonadotropin (hCG), a glycoprotein hormone essential for pregnancy maintenance, that has also been involved in angiogenesis and maternal immunotolerance [53,54]. Some studies have reported associations between adverse pregnancy outcomes like preeclampsia and circulating levels of PSGs and hCG [52,53,55,56]. Importantly, PSG3 and CGB3 are involved in trophoblast syncytialization; alterations in expression of these genes have been described in other viral infections [57,58]. However, to our knowledge, there are no studies linking past maternal SARS-CoV-2 infection to altered placental PSG3 or CGB3 expression or trophoblast differentiation. Yet at least one report has linked Covid-19 with decreased in expression of organ-specific cell type transcripts in multiple organs [59].
We also observed increased expression of CXCL10 in placentas delivered from participants exposed to SARS-CoV-2 infection during pregnancy. CXCL10 (C-X-C motif chemokine ligand 10) is a pro-inflammatory chemokine secreted in response to interferon gamma (IFNγ) involved in the stimulation of monocytes, natural killer, and T-cells. Previous investigations have also reported CXCL10 gene expression upregulation in bronchoalveolar lavages [60], nasopharyngeal swabs [61] and male placentas exposed to maternal SARS-CoV-2 infection [43]. Importantly, CXCL10 may also be a key regulator of the “cytokine storm” in response to SARS-CoV-2 infection and circulating levels of CXCL10 are reported to be positively associated with disease severity [[62], [63], [64]]. Two other immune genes, TLR3 and DDX58, were overexpressed in placentas delivered from SARS-CoV-2 IgG positive participants. TLR3 is a member of the toll-like receptor (TLR) family and DDX58 encodes a protein containing RNA helicase-DEAD box motifs (also known as RIG-I). These genes are involved in recognizing double-stranded RNA (dsRNA) released during viral replication and activation of the innate immune response [65,66]. To date, placental expression of these genes has not been linked to SARS-CoV-2 infection. However, peripheral blood TLR3 gene expression is reduced in patients with severe COVID-19 compared to those with mild forms of the disease [67] and DDX58 expression in human lung cells has been implicated in the initial response against SARS-CoV-2 infection [68,69].
We also detected a slight decrease EGR1 placental gene expression in pregnancies exposed to SARS-CoV-2 infection compared to controls. EGR1 encodes a zinc-finger transcription factor that represses the activity of inflammatory gene enhancers in macrophages [70]. A recent study showed increased expression of EGR1 in blood of from patients with Covid-19 [71]. In the placenta, EGR1 expression has been associated with maternal obesity [72]. However, to our knowledge, no studies to date reporting specifically on EGR1 placental expression and Covid-19.
Strengths of the Generation C study include a demographically diverse population of pregnant participants recruited in NYC after the start of the SARS-CoV-2 pandemic. To assess SARS-CoV-2 IgG levels, we used a highly sensitive (95%) and specific (100%) serological assay. Also, we explored the effects of SARS-CoV-2 IgG seropositivity on placental histopathology and gene expression simultaneously. We acknowledge that our study is not without limitations. The sample size is limited, and the number of placentas delivered from SARS-CoV-2 IgG positive participants is small. We do not have information on the precise timing of SARS-CoV-2 infection, disease severity, or newborn SARS-CoV-2 IgG levels. Given the exploratory nature of this study, we only measured expression of a small number of genes, and we did not account for multiple testing. Larger studies are needed to investigate the effects of SARS-CoV-2 infection on the whole placental transcriptome. In summary, we found evidence of an association between SARS-CoV-2 infection during pregnancy and placental expression of trophoblast and immune related genes which could potentially contribute to long-term health effects in the offspring. Future research should confirm the observed associations and assess potential long-term implications.
Funding
The Generation C cohort was established through funding from the US Centers for Disease Control and Prevention (CDC), who also provided technical assistance related to analysis and interpretation of data and writing the report (contract 75D30120C08186). CL is funded through the US National Institutes of Health [NIH/ NICHD R00HD097286]. The findings and conclusions in this report are those of the authors and do not necessarily represent the position of the funding agencies. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Declaration of competing interest
Dr. Florian Krammer (a co-investigator of the Ceneration C study) is a co-inventor of assays that test for COVID-19 antibodies and antigens. Patents for these assays have been filed through the Icahn School of Medicine at Mount Sinai and one of these antibody assays is currently licensed to commercial entities, including Kantaro Biosciences, a Mount Sinai spin-out company. The medical school has and will receive payments related to commercialization of these assays and as an inventor, Dr. Krammer is entitled to a portion of these payments. Dr. Krammer is also a co-inventor of a novel COVID-19 vaccine currently being investigated in clinical trials.
Acknowledgements
We would like to thank all participants of the Generation C study for their cooperation and contribution to the research field. We would like to thank the members of the Krammer Serology Core Study group and the Mount Sinai Biorepository and Pathology Core, and especially Maryann Huie, Frances Avila, Ariane Benedetto, Anastasiya Dzhun.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.placenta.2022.06.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan A., Hamilton J.P., Alqahtani S.A., T A.W. A narrative review of coronavirus disease 2019 (COVID-19): clinical, epidemiological characteristics, and systemic manifestations. Intern. Emerg. Med. 2021;16(4):815–830. doi: 10.1007/s11739-020-02616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020:1–8. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mor G., Aldo P., Alvero A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017;17(8):469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 8.Villar J., Ariff S., Gunier R.B., Thiruvengadam R., Rauch S., Kholin A., Roggero P., Prefumo F., do Vale M.S., Cardona-Perez J.A., Maiz N., Cetin I., Savasi V., Deruelle P., Easter S.R., Sichitiu J., Soto Conti C.P., Ernawati E., Mhatre M., Teji J.S., Liu B., Capelli C., Oberto M., Salazar L., Gravett M.G., Cavoretto P.I., Nachinab V.B., Galadanci H., Oros D., Ayede A.I., Sentilhes L., Bako B., Savorani M., Cena H., García-May P.K., Etuk S., Casale R., Abd-Elsalam S., Ikenoue S., Aminu M.B., Vecciarelli C., Duro E.A., Usman M.A., John-Akinola Y., Nieto R., Ferrazi E., Bhutta Z.A., Langer A., Kennedy S.H., Papageorghiou A.T. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., Woodworth K.R., Nahabedian J.F., III, Azziz-Baumgartner E., Gilboa S.M. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69(44):1641. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papageorghiou A.T., Deruelle P., Gunier R.B., Rauch S., García-May P.K., Mhatre M., Usman M.A., Abd-Elsalam S., Etuk S., Simmons L.E., Napolitano R., Deantoni S., Liu B., Prefumo F., Savasi V., do Vale M.S., Baafi E., Zainab G., Nieto R., Maiz N., Aminu M.B., Cardona-Perez J.A., Craik R., Winsey A., Tavchioska G., Bako B., Oros D., Rego A., Benski A.C., Hassan-Hanga F., Savorani M., Giuliani F., Sentilhes L., Risso M., Takahashi K., Vecchiarelli C., Ikenoue S., Thiruvengadam R., Soto Conti C.P., Ferrazzi E., Cetin I., Nachinab V.B., Ernawati E., Duro E.A., Kholin A., Firlit M.L., Easter S.R., Sichitiu J., Bowale A., Casale R., Cerbo R.M., Cavoretto P.I., Eskenazi B., Thornton J.G., Bhutta Z.A., Kennedy S.H., Villar J. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 2021;225(3) doi: 10.1016/j.ajog.2021.05.014. 289.e1-289.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurol-Urganci I., Jardine J.E., Carroll F., Draycott T., Dunn G., Fremeaux A., Harris T., Hawdon J., Morris E., Muller P., Waite L., Webster K., van der Meulen J., Khalil A. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karasek D., Baer R.J., McLemore M.R., Bell A.J., Blebu B.E., Casey J.A., Coleman-Phox K., Costello J.M., Felder J.N., Flowers E. The Lancet Regional Health-Americas; 2021. The Association of COVID-19 Infection in Pregnancy with Preterm Birth: A Retrospective Cohort Study in California. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., Pomar L. Second-Trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020 doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacinti K.G., Kalafat E., Sukur Y.E., Koc A. Increased incidence of first-trimester miscarriage during the COVID-19 pandemic. Ultrasound Obstet. Gynecol. 2021;57(6):1013–1014. doi: 10.1002/uog.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazemi S.N., Hajikhani B., Didar H., Hosseini S.S., Haddadi S., Khalili F., Mirsaeidi M., Nasiri M.J. COVID-19 and cause of pregnancy loss during the pandemic: a systematic review. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conde-Agudelo A., Romero R. American Journal of Obstetrics and Gynecology; 2021. SARS-CoV-2 Infection during Pregnancy and Risk of Preeclampsia: a Systematic Review and Meta-Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSisto C.L., Wallace B., Simeone R.M., Polen K., Ko J.Y., Meaney-Delman D., Ellington S.R. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-september 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70(47):1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ (Can. Med. Assoc. J.) 2021;193(16) doi: 10.1503/cmaj.202604. E540-e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molenaar N.M., Rommel A.S., de Witte L., Dolan S.M., Lieb W., Ibroci E., Ohrn S., Lynch J., Capuano C., Stadlbauer D., Krammer F., Zapata L.B., Brody R.I., Pop V.J., Jessel R.H., Sperling R.S., Afzal O., Gigase F., Missall R., Janevic T., Stone J., Howell E.A., Bergink V. Paediatr Perinat Epidemiol; 2021. SARS-CoV-2 during Pregnancy and Associated Outcomes: Results from an Ongoing Prospective Cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egerup P., Fich Olsen L., Christiansen A.H., Westergaard D., Severinsen E.R., Hviid K.V.R., Kolte A.M., Boje A.D., Bertelsen M.M.F., Prætorius L., Zedeler A., Nielsen J.R., Bang D., Berntsen S., Ethelberg-Findsen J., Storm D.M., Bello-Rodríguez J., Ingham A., Ollé-López J., Hoffmann E.R., Wilken-Jensen C., Krebs L., Jørgensen F.S., Westh H., Jørgensen H.L., la Cour Freiesleben N., Nielsen H.S. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies at delivery in women, partners, and newborns. Obstet. Gynecol. 2021;137(1):49–55. doi: 10.1097/AOG.0000000000004199. [DOI] [PubMed] [Google Scholar]
- 21.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pique-Regi R., Romero R., Tarca A.L., Luca F., Xu Y., Alazizi A., Leng Y., Hsu C.D., Gomez-Lopez N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife. 2020;9 doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashary N., Bhide A., Chakraborty P., Colaco S., Mishra A., Chhabria K., Jolly M.K., Modi D. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 2020;8:783. doi: 10.3389/fcell.2020.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu-Culligan A., Chavan A.R., Vijayakumar P., Irshaid L., Courchaine E.M., Milano K.M., Tang Z., Pope S.D., Song E., Vogels C.B.F., Lu-Culligan W.J., Campbell K.H., Casanovas-Massana A., Bermejo S., Toothaker J.M., Lee H.J., Liu F., Schulz W., Fournier J., Muenker M.C., Moore A.J., Konnikova L., Neugebauer K.M., Ring A., Grubaugh N.D., Ko A.I., Morotti R., Guller S., Kliman H.J., Iwasaki A., Farhadian S.F. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Medicine. 2021;2(5):591–610. doi: 10.1016/j.medj.2021.04.016. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahmi A., Brügger M., Démoulins T., Zumkehr B., Oliveira Esteves B.I., Bracher L., Wotzkow C., Blank F., Thiel V., Baud D., Alves M.P. SARS-CoV-2 can infect and propagate in human placenta explants. Cell. Rep. Med. 2021 doi: 10.1016/j.xcrm.2021.100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma S., Joshi C.S., Silverstein R.B., He M., Carter E.B., Mysorekar I.U. SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system. Medicine. 2021;2(5):575–590. doi: 10.1016/j.medj.2021.04.009. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taglauer E.S., Wachman E.M., Juttukonda L., Klouda T., Kim J., Wang Q., Ishiyama A., Hackam D.J., Yuan K., Jia H. Acute SARS-CoV-2 infection in pregnancy is associated with placental ACE-2 shedding. bioRxiv. 2021 doi: 10.1016/j.ajpath.2021.12.011. 2021.11.19.469335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., Zhou W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol. 2020;37(8):861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., Benachi A., De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., Yasumoto Y., Vogels C.B., Casanovas-Massana A., Vijayakumar P., Geng B., Odio C.D., Fournier J., Brito A.F., Fauver J.R., Liu F., Alpert T., Tal R., Szigeti-Buck K., Perincheri S., Larsen C., Gariepy A.M., Aguilar G., Fardelmann K.L., Harigopal M., Taylor H.S., Pettker C.M., Wyllie A.L., Cruz C.D., Ring A.M., Grubaugh N.D., Ko A.I., Horvath T.L., Iwasaki A., Reddy U.M., Lipkind H.S. SARS-CoV-2 infection of the placenta. J. Clin. Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J. Obstet. Gynecol. MFM. 2020;2(3) doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debelenko L., Katsyv I., Chong A.M., Peruyero L., Szabolcs M., Uhlemann A.C. Trophoblast damage with acute and chronic intervillositis: disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum. Pathol. 2021;109:69–79. doi: 10.1016/j.humpath.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong L., Pei S., Ren Q., Fu S., Yu L., Chen H., Chen X., Yin M. Evaluation of vertical transmission of SARS-CoV-2 in utero: nine pregnant women and their newborns. Placenta. 2021;111:91–96. doi: 10.1016/j.placenta.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitan D., London V., McLaren R.A., Mann J.D., Cheng K., Silver M., Balhotra K.S., McCalla S., Loukeris K. Histologic and immunohistochemical evaluation of 65 placentas from women with polymerase chain reaction-proven severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Arch. Pathol. Lab Med. 2021;145(6):648–656. doi: 10.5858/arpa.2020-0793-SA. [DOI] [PubMed] [Google Scholar]
- 37.Resta L., Vimercati A., Cazzato G., Mazzia G., Cicinelli E., Colagrande A., Fanelli M., Scarcella S.V., Ceci O., Rossi R. SARS-CoV-2 and placenta: new insights and perspectives. Viruses. 2021;13(5) doi: 10.3390/v13050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertero L., Borella F., Botta G., Carosso A., Cosma S., Bovetti M., Carosso M., Abbona G., Collemi G., Papotti M., Cassoni P., Benedetto C. Placenta histopathology in SARS-CoV-2 infection: analysis of a consecutive series and comparison with control cohorts. Virchows Arch. 2021:1–14. doi: 10.1007/s00428-021-03097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hecht J.L., Quade B., Deshpande V., Mino-Kenudson M., Ting D.T., Desai N., Dygulska B., Heyman T., Salafia C., Shen D., Bates S.V., Roberts D.J. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020;33(11):2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts D.J., Edlow A.G., Romero R.J., Coyne C.B., Ting D.T., Hornick J.L., Zaki S.R., Das Adhikari U., Serghides L., Gaw S.L., Metz T.D. A standardized definition of placental infection by SARS-CoV-2, a consensus statement from the national institutes of health/eunice kennedy shriver national institute of child health and human development SARS-CoV-2 placental infection Workshop. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girolamo R.D., Khalil A., Alameddine S., D'Angelo E., Galliani C., Matarrelli B., Buca D., Liberati M., Rizzo G., D'Antonio F. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J. Obstet. Gynecol. MFM. 2021 doi: 10.1016/j.ajogmf.2021.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., Gismondo M.R., Perotti F., Callegari C., Mancon A., Cammarata S., Beretta I., Nebuloni M., Trabattoni D., Clerici M., Savasi V. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020;11(1):5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bordt E.A., Shook L.L., Atyeo C., Pullen K.M., De Guzman R.M., Meinsohn M.C., Chauvin M., Fischinger S., Yockey L.J., James K., Lima R., Yonker L.M., Fasano A., Brigida S., Bebell L.M., Roberts D.J., Pépin D., Huh J.R., Bilbo S.D., Li J.Z., Kaimal A., Schust D.J., Gray K.J., Lauffenburger D., Alter G., Edlow A.G. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci. Transl. Med. 2021;13(617) doi: 10.1126/scitranslmed.abi7428. eabi7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Flores V., Romero R., Xu Y., Theis K.R., Arenas-Hernandez M., Miller D., Peyvandipour A., Bhatti G., Galaz J., Gershater M., Levenson D., Pusod E., Tao L., Kracht D., Florova V., Leng Y., Motomura K., Para R., Faucett M., Hsu C.D., Zhang G., Tarca A.L., Pique-Regi R., Gomez-Lopez N. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 2022;13(1):320. doi: 10.1038/s41467-021-27745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldstein J.A., Gallagher K., Beck C., Kumar R., Gernand A.D. Maternal-fetal inflammation in the placenta and the developmental origins of health and disease. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.531543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A., Tan J., Bhavsar D., Capuano C., Kirkpatrick E., Meade P., Brito R.N., Teo C., McMahon M., Simon V., Krammer F. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol. 2020;57(1) doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khong T.Y., Mooney E.E., Ariel I., Balmus N.C., Boyd T.K., Brundler M.A., Derricott H., Evans M.J., Faye-Petersen O.M., Gillan J.E., Heazell A.E., Heller D.S., Jacques S.M., Keating S., Kelehan P., Maes A., McKay E.M., Morgan T.K., Nikkels P.G., Parks W.T., Redline R.W., Scheimberg I., Schoots M.H., Sebire N.J., Timmer A., Turowski G., van der Voorn J.P., van Lijnschoten I., Gordijn S.J. Sampling and definitions of placental lesions: Amsterdam placental Workshop group consensus statement. Arch. Pathol. Lab Med. 2016;140(7):698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 48.Waggott D., Chu K., Yin S., Wouters B.G., Liu F.F., Boutros P.C. NanoStringNorm: an extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics. 2012;28(11):1546–1548. doi: 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7) doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Team R.C., R . R Foundation for Statistical Computing; Vienna, Austria: 2021. A Language and Environment for Statistical Computing. [Google Scholar]
- 51.Suhren J.T., Meinardus A., Hussein K., Schaumann N. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta. 2021;117:72–77. doi: 10.1016/j.placenta.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teglund S., Olsen A., Khan W.N., Frängsmyr L., Hammarström S. The pregnancy-specific glycoprotein (PSG) gene cluster on human chromosome 19: fine structure of the 11 PSG genes and identification of 6 new genes forming a third subgroup within the carcinoembryonic antigen (CEA) family. Genomics. 1994;23(3):669–684. doi: 10.1006/geno.1994.1556. [DOI] [PubMed] [Google Scholar]
- 53.Fournier T., Guibourdenche J., Evain-Brion D. Review: hCGs: different sources of production, different glycoforms and functions. Placenta. 2015;36(Suppl 1):S60–S65. doi: 10.1016/j.placenta.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Schumacher A., Zenclussen A.C. Human chorionic gonadotropin-mediated immune responses that facilitate embryo implantation and placentation. Front. Immunol. 2019;10:2896. doi: 10.3389/fimmu.2019.02896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore T., Dveksler G.S. Pregnancy-specific glycoproteins: complex gene families regulating maternal-fetal interactions. Int. J. Dev. Biol. 2014;58(2–4):273–280. doi: 10.1387/ijdb.130329gd. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann W., Kammerer R. The immune-modulating pregnancy-specific glycoproteins evolve rapidly and their presence correlates with hemochorial placentation in primates. BMC Genom. 2021;22(1):128. doi: 10.1186/s12864-021-07413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mimura N., Nagamatsu T., Morita K., Taguchi A., Toya T., Kumasawa K., Iriyama T., Kawana K., Inoue N., Fujii T., Osuga Y. Suppression of human trophoblast syncytialization by human cytomegalovirus infection. Placenta. 2022;117:200–208. doi: 10.1016/j.placenta.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Fisher S., Genbacev O., Maidji E., Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 2000;74(15):6808–6820. doi: 10.1128/jvi.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park J., Foox J., Hether T., Danko D.C., Warren S., Kim Y., Reeves J., Butler D.J., Mozsary C., Rosiene J., Shaiber A., Afshin E.E., MacKay M., Rendeiro A.F., Bram Y., Chandar V., Geiger H., Craney A., Velu P., Melnick A.M., Hajirasouliha I., Beheshti A., Taylor D., Saravia-Butler A., Singh U., Wurtele E.S., Schisler J., Fennessey S., Corvelo A., Zody M.C., Germer S., Salvatore S., Levy S., Wu S., Tatonetti N.P., Shapira S., Salvatore M., Westblade L.F., Cushing M., Rennert H., Kriegel A.J., Elemento O., Imielinski M., Rice C.M., Borczuk A.C., Meydan C., Schwartz R.E., Mason C.E. System-wide transcriptome damage and tissue identity loss in COVID-19 patients. Cell. Rep. Med. 2022;3(2) doi: 10.1016/j.xcrm.2022.100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grant R.A., Morales-Nebreda L., Markov N.S., Swaminathan S., Querrey M., Guzman E.R., Abbott D.A., Donnelly H.K., Donayre A., Goldberg I.A., Klug Z.M., Borkowski N., Lu Z., Kihshen H., Politanska Y., Sichizya L., Kang M., Shilatifard A., Qi C., Lomasney J.W., Argento A.C., Kruser J.M., Malsin E.S., Pickens C.O., Smith S.B., Walter J.M., Pawlowski A.E., Schneider D., Nannapaneni P., Abdala-Valencia H., Bharat A., Gottardi C.J., Budinger G.R.S., Misharin A.V., Singer B.D., Wunderink R.G. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021;590(7847):635–641. doi: 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butler D., Mozsary C., Meydan C., Foox J., Rosiene J., Shaiber A., Danko D., Afshinnekoo E., MacKay M., Sedlazeck F.J., Ivanov N.A., Sierra M., Pohle D., Zietz M., Gisladottir U., Ramlall V., Sholle E.T., Schenck E.J., Westover C.D., Hassan C., Ryon K., Young B., Bhattacharya C., Ng D.L., Granados A.C., Santos Y.A., Servellita V., Federman S., Ruggiero P., Fungtammasan A., Chin C.S., Pearson N.M., Langhorst B.W., Tanner N.A., Kim Y., Reeves J.W., Hether T.D., Warren S.E., Bailey M., Gawrys J., Meleshko D., Xu D., Couto-Rodriguez M., Nagy-Szakal D., Barrows J., Wells H., O'Hara N.B., Rosenfeld J.A., Chen Y., Steel P.A.D., Shemesh A.J., Xiang J., Thierry-Mieg J., Thierry-Mieg D., Iftner A., Bezdan D., Sanchez E., Campion T.R., Jr., Sipley J., Cong L., Craney A., Velu P., Melnick A.M., Shapira S., Hajirasouliha I., Borczuk A., Iftner T., Salvatore M., Loda M., Westblade L.F., Cushing M., Wu S., Levy S., Chiu C., Schwartz R.E., Tatonetti N., Rennert H., Imielinski M., Mason C.E. Shotgun transcriptome, spatial omics, and isothermal profiling of SARS-CoV-2 infection reveals unique host responses, viral diversification, and drug interactions. Nat. Commun. 2021;12(1):1660. doi: 10.1038/s41467-021-21361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coperchini F., Chiovato L., Rotondi M. Interleukin-6, CXCL10 and infiltrating macrophages in COVID-19-related cytokine storm: not one for all but all for one. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.668507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blot M., Bour J.B., Quenot J.P., Bourredjem A., Nguyen M., Guy J., Monier S., Georges M., Large A., Dargent A., Guilhem A., Mouries-Martin S., Barben J., Bouhemad B., Charles P.E., Chavanet P., Binquet C., Piroth L. The dysregulated innate immune response in severe COVID-19 pneumonia that could drive poorer outcome. J. Transl. Med. 2020;18(1):457. doi: 10.1186/s12967-020-02646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorè N.I., De Lorenzo R., Rancoita P.M.V., Cugnata F., Agresti A., Benedetti F., Bianchi M.E., Bonini C., Capobianco A., Conte C., Corti A., Furlan R., Mantegani P., Maugeri N., Sciorati C., Saliu F., Silvestri L., Tresoldi C., Ciceri F., Rovere-Querini P., Di Serio C., Cirillo D.M., Manfredi A.A. CXCL10 levels at hospital admission predict COVID-19 outcome: hierarchical assessment of 53 putative inflammatory biomarkers in an observational study. Mol. Med. 2021;27(1):129. doi: 10.1186/s10020-021-00390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 66.Rehwinkel J., Gack M.U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020;20(9):537–551. doi: 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menezes M.C.S., Veiga A.D.M., Martins de Lima T., Kunimi Kubo Ariga S., Vieira Barbeiro H., de Lucena Moreira C., Pinto A.A.S., Brandao R.A., Marchini J.F., Alencar J.C., Marino L.O., Gomez L.M., Olsen Saraiva Camara N., Souza H.P. Lower peripheral blood Toll-like receptor 3 expression is associated with an unfavorable outcome in severe COVID-19 patients. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-94624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamada T., Sato S., Sotoyama Y., Orba Y., Sawa H., Yamauchi H., Sasaki M., Takaoka A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021;22(7):820–828. doi: 10.1038/s41590-021-00942-0. [DOI] [PubMed] [Google Scholar]
- 69.Yin X., Riva L., Pu Y., Martin-Sancho L., Kanamune J., Yamamoto Y., Sakai K., Gotoh S., Miorin L., De Jesus P.D., Yang C.C., Herbert K.M., Yoh S., Hultquist J.F., García-Sastre A., Chanda S.K. MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep. 2021;34(2) doi: 10.1016/j.celrep.2020.108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trizzino M., Zucco A., Deliard S., Wang F., Barbieri E., Veglia F., Gabrilovich D., Gardini A. EGR1 is a gatekeeper of inflammatory enhancers in human macrophages. Sci. Adv. 2021;7(3) doi: 10.1126/sciadv.aaz8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X.N., You Y., Cui X.M., Gao H.X., Wang G.L., Zhang S.B., Yao L., Duan L.J., Zhu K.L., Wang Y.L., Li L., Lu J.H., Wang H.B., Fan J.F., Zheng H.W., Dai E.H., Tian L.Y., Ma M.J. Single-cell immune profiling reveals distinct immune response in asymptomatic COVID-19 patients. Signal Transduct. Targeted Ther. 2021;6(1):342. doi: 10.1038/s41392-021-00753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saben J., Lindsey F., Zhong Y., Thakali K., Badger T.M., Andres A., Gomez-Acevedo H., Shankar K. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–177. doi: 10.1016/j.placenta.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.