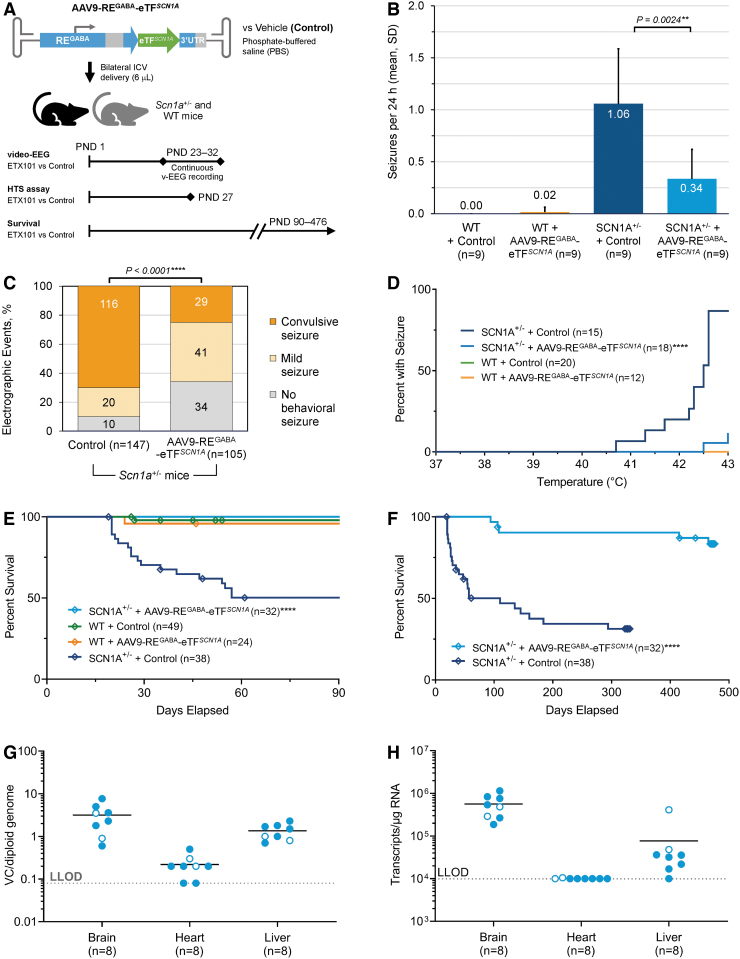
Figure 4.
AAV9-REGABA-eTFSCN1A reduces frequency and severity of spontaneous seizures, protects against febrile seizures, and demonstrates durable survival efficacy and persistent activity for up to 470 days postdosing in Scn1a+/− mice. (A) Study design. AAV9-REGABA-eTFSCN1A or vehicle alone (PBS) were administered by bilateral ICV injection (3 μL/hemisphere) to heterozygous Scn1a+/− or WT littermates at PND1. Separate studies assessed electrographic seizure monitoring of spontaneous seizures (following doses of 1.2–5.4E11 vg/animala vs. vehicle); susceptibility to HTS (3.7E10 vg/animala vs. vehicle); and survival and long-term persistence of AAV9-REGABA-eTFSCN1A (3.7E10 vg/animala vs. vehicle). At 470 days, the animals were sacrificed, and brain, heart, and liver tissues were evaluated for AAV9-REGABA-eTFSCN1A VCN by ddPCR and eTF transgene mRNA transcript levels by RT-ddPCR. (B) EEG seizure frequency. Mice treated with AAV9-REGABA-eTFSCN1A experienced a 68% reduction in the mean daily generalized seizure frequency per animal compared with Scn1a+/− controls (**p = 0.0024, unpaired t-test). No changes in seizure frequency were detected in WT mice treated with AAV9-REGABA-eTFSCN1A. (C) EEG seizure severity. Video characterization of all recorded electrographic seizure events revealed that treatment of Scn1a+/− mice with AAV9-REGABA-eTFSCN1A significantly reduced convulsive (tonic-clonic) seizures compared with Scn1a+/− mice treated with vehicle (****p < 0.0001; chi-square test). Most events detected in AAV9-REGABA-eTFSCN1A-treated Scn1a+/– mice were mild (characterized by mild movements and/or head twitching, but without convulsions) or nonbehavioral seizures. (D) HTS assay. Percentage of AAV9-REGABA-eTFSCN1A PND1-treated Scn1a+/− mice experiencing seizures at a given temperature at PND27. WT groups overlap along the zero line (green and orange lines; only the orange trace is shown). p-Values calculated using a Log-rank test, ****p < 0.0001. (E) Ninety-day survival. The 90-day survival rate in AAV9-REGABA-eTFSCN1A-treated Scn1a+/– mice was 100% compared with 50% in control-treated Scn1a+/− mice (****p < 0.0001, Log-rank test). No difference in survival was observed between WT mice ± AAV9-REGABA-eTFSCN1A and Scn1a+/− mice + AAV9-REGABA-eTFSCN1A. (F) Long-term Survival. Long-term follow-up showed that survival benefit of AAV9-REGABA-eTFSCN1A was sustained over ∼470 days after dosing (****p < 0.0001, Log-rank test). Diamond indicates humane endpoint or end of study euthanasia at approximately day 330 for vehicle and day 470 for AAV9-REGABA-eTFSCN1A-treated animals. (G) VCN biodistribution in Scn1a+/− mice administered AAV9-REGABA-eTFSCN1A. At 470 days, the animals were sacrificed, and brain, heart, and liver tissues were evaluated for AAV9-REGABA-eTFSCN1A VCN by ddPCR and eTFSCN1A transgene mRNA transcripts levels by RT-ddPCR. (H) eTFSCN1A mRNA expression levels in Scn1a+/− mice administered AAV9-REGABA-eTFSCN1A. eTFSCN1A mRNA transcripts per microgram of RNA analyzed. Seven of eight animals had no measurable eTFSCN1A mRNA transcripts in the heart. Open circle indicates female gender. Dotted line indicates the limit of detection in both assays. aDoses in the EEG study were determined by a qPCR titering method while the HTS and survival studies were titered via ddPCR; thus the doses used across studies are not directly comparable. EEG, electroencephalography; HTS, hyperthermia-induced seizure; LLOD, lower limit of detection; qPCR, quantitative polymerase chain reaction; RT-ddPCR, reverse transcription digital droplet polymerase chain reaction; VCN, vector copy number.