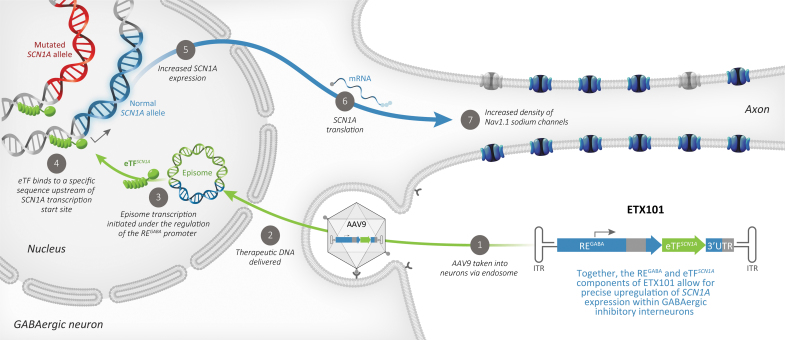
Figure 6.
Mechanism of action of AAV9-REGABA-eTFSCN1A (ETX101), a cell-selective AAV-mediated SCN1A gene regulation therapy that upregulates expression of WT SCN1A selectively in GABAergic inhibitory interneurons to compensate for the loss-of-function mutant alleles in individuals with DS. As previously described,46 AAV vector enters the cell by endocytosis in a receptor-mediated manner. Vector escapes from the endosome, enters the nucleus through the nuclear pore complex, and predominantly exists as a nonreplicating episome. Episome transcription is then initiated under the regulation of the REGABA promoter to produce eTFSCN1A preferably in GABAergic neurons. eTFSCN1A binds to a conserved regulatory region upstream of the SCN1A transcription start site, promoting increased SCN1A expression and protein translation, thereby increasing the density of membrane-associated NaV1.1 sodium channels and restoring function. While eTFSCN1A also binds the mutated SCN1A allele, it does not produce any stable protein capable of functioning at the neuronal membrane. This approach leverages natural patterns of gene expression to increase production of NaV1.1 protein and restore inhibitory function while minimizing potential off target effects. DS, Dravet syndrome; RE, regulatory element.