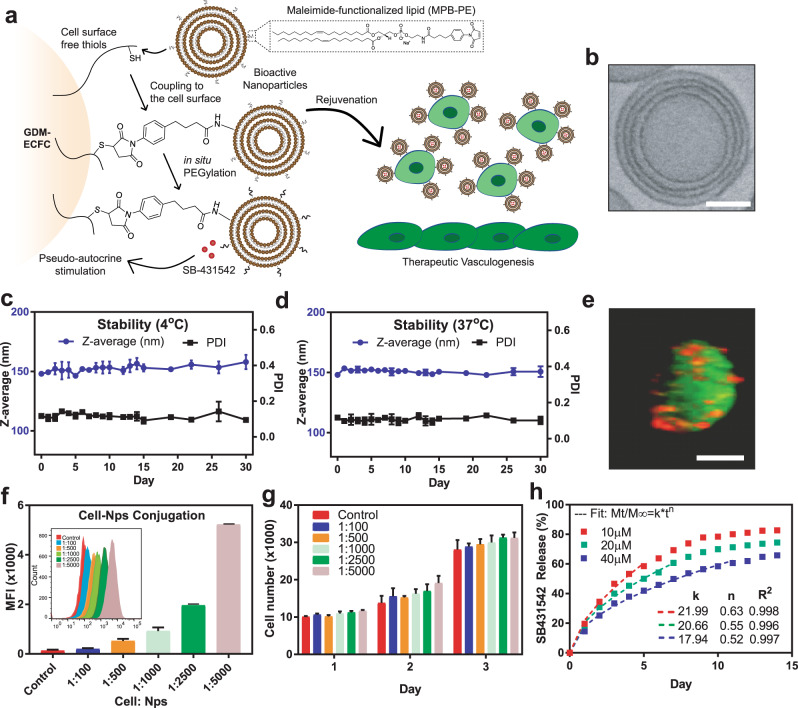
Fig. 1. Synthesis and characterization of bioactive nanoparticles.
a Schematic of maleimide-based conjugation of bioactive nanoparticles to the ECFC surface’s free thiols followed by in situ PEGylation. Continuous pseudo-autocrine stimulation of GDM-ECFCs with SB-431542 improves their clinical potential for therapeutic vasculogenesis. b A CryoTEM photograph demonstrating the multilamellar structure of the nanoparticle. Scale bar is 50 nm. c, d The stability of the nanoparticles was quantified using DLS for hydrodynamic diameter (z-average) and polydispersity (PDI) over 30 days in buffer at (c) 4 °C and (d) 37 °C (physiological temperature). The data represent the mean ± s.d. of three independent experiments conducted in triplicate. e A confocal photograph demonstrating a stable conjugation of Dil-labeled multilamellar lipid nanoparticles (red) conjugated onto the surface of a CFSE-labeled ECFC (green). Scale bar is 10 µm. f Flow cytometry analysis demonstrated an increase in mean fluorescence intensity (MFI) when the ratio of cell to nanoparticles was increased from 1:100 to 1:5,000. Inset indicates the corresponding histogram data. g The viability of ECFCs with various cell to nanoparticle ratios (1:100 to 1:5,000) was quantified using alamar blue assay over three days. No significant difference was observed between non-conjugated cells and nanoparticle-conjugated cells (mean ± S.D., three independent experiments conducted in triplicate). h Accumulative release of the bioactive small molecule, SB-431542 (SB), from the NPs over 2 weeks. Three different initial concentrations of SB (10, 20, 40 µM) were encapsulated into the NPs and the amounts of SB released were measured daily. The release profiles were fit for Korsmeyer-Peppas equation and the fitting parameters are shown within the graph.