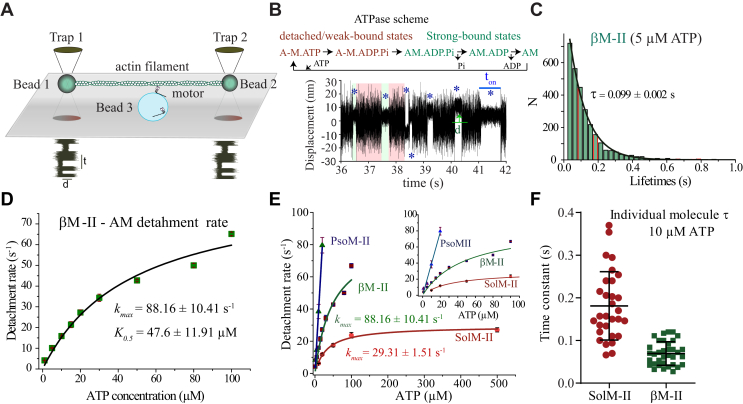
Figure 2.
ATP concentration dependence of AM bound lifetimes for βM-II and SolM-II.A, optical trap setup for three-bead assay. Note that the various components of the setup are not drawn to scale. B, original data trace displaying the bead position signal over time. Example shows single myosin molecule interacting with an actin filament at 5 μM ATP. Several AM-interaction events are indicated with blue asterix. ton is the duration of AM association. The green arrow illustrates the displacement (d) from average unbound to bound position. The AM bound and unbound states are depicted in simplified ATPase scheme and corresponding signal change is shown with green and red background on the data trace, respectively. C, AM-interaction events ton measured at 5 μM ATP are plotted in a histogram. The average lifetimes (τ) were calculated by least-squares fitting of a histogram with single exponential decay function. D, the AM detachment rate is the inverse of AM-bound average lifetime (τ). Detachment rates (1/τ) at increasing concentration of ATP from 1 to 100 μM derived from τ as shown in (C) are plotted for βM-II. ATP concentration dependence of the detachment rate follows the Michaelis–Menten kinetics and thus fitted with the function, v = Vmax∗x/(Km + x). Maximum detachment rate (Vmax or kmax) and ATP concentration at ½ kmax, that is, Km (or K0.5) was derived, R2 = 0.97. E, ATP concentration dependence of the AM-detachment rate (1/τ) as a function of ATP concentration are compared for βM-II, SolM-II, and PsoM-II. Inset shows the clear kinetic difference observed at lower ATP concentrations among the three myosin forms. Rates for βM-II and SolM-II fitted with Michaelis–Menten function and for PsoM-II, with linear regression. For PsoM-II, kT from linear regression was 3.96 ± 0.31 μM−1 s−1; for βM-II, kmax = 88.16 ± 10 s−1, K0.5 = 47.6 ± 11 μM; for SolM-II, kmax = 29.3 ± 1.5 s−1, K0.5 = 31.6 ± 3.7 μM. F, scatter plot depicts the time constants determined from measurements at 10 μM ATP from individual molecules for SolM-II (N = 30) and βM-II (N = 35) and are significantly different p < 0.0001 (two-tailed t test). The error bars are average ± SD. Altogether, 231 individual βM-II molecules and 36,000 AM-binding events were identified and analyzed. At 1 μM ATP, N = 59, n = 8118; 5 μM ATP, N = 18, n = 3002; 10 μM ATP, N = 48, n = 8164; 15 μM ATP, N = 21, n = 2699; 20 μM ATP, N = 22, n = 3893; 30 μM ATP, N = 10, n = 1050; at 50 μM ATP, N = 33, n = 5375; 80 μM ATP, N = 14, n = 4659; at 100 μM ATP, N = 15, n = 1381. The single-molecule experiments were performed with myosins from at least three separate βM-II preparations. Note that event lifetime measurements at 50, 80, and 100 μM ATP were measured by applying the fast triangular wave of 600 Hz on one of the beads so that the binding events are discernible in the data records. For SolM-II, 10 μM ATP N = 30, n = 4344; 20 μM ATP N = 53, n = 7336; 50 μM ATP, N = 16, n = 1651; 100 μM ATP, N = 40, n = 4937; 500 μM ATP, N = 40, n = 2099. For PsoM-II, in total 30 individual myosin molecules were measured for 1, 5, 10, and 20 μM ATP conditions. N = number of individual myosin molecules and n = number of AM-association events. The event lifetimes for βM-II and SolM-II were compared between different ATP concentrations using the nonparametric Mann–Whitney U test, which yielded the statistical differences in ton with p < 0.0001. Note that the detachment rates for PsoM-II in Figure 2E, which is used for comparison are taken from our previous publication and reused with permission from (23) Copyright © 2020, American Chemical Society. AM, actomyosin.