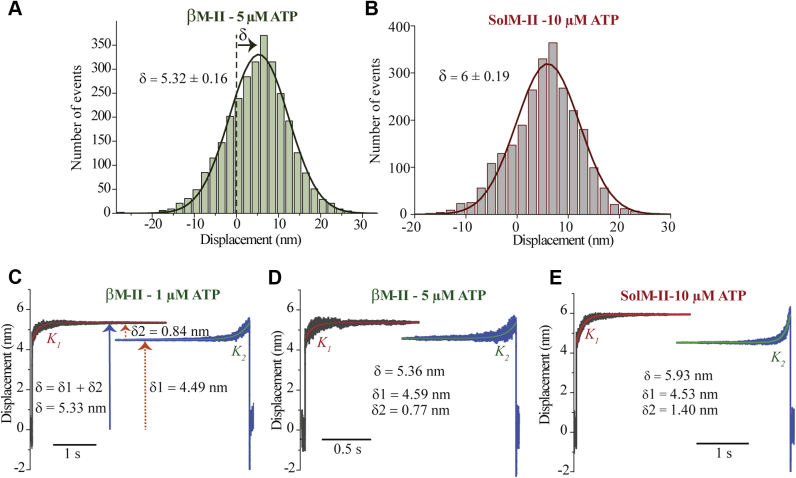
Figure 3.
Power stroke size of β-cardiac myosin. A and B, the histogram with displacement events measured at 5 μM ATP for βMII and at 10 μM ATP for SolM-II. The average stroke size was estimated by using shift-of-histogram method (74). Least squares fitting of event distribution with Gaussian function yields average power stroke, δ = 5.32 ± 0.16 nm and 6 ± 0.19 nm for βM-II and SolM-II, respectively. p ˂ 0.05 with unpaired t test. B–E, ensemble averaging of the individual AM-association events. The beginning and the end of the several individual AM-binding events were synchronized and fitted with single exponential functions to estimate the substeps. The beginning is shown as a forward fit (red points) and the end (green points) as a backward fit. Average stroke size (δ) and displacement size corresponding to the first and second stroke is indicated as δ1 and δ2, respectively. K1—reaction rate for the ADP dissociation and K2—ATP induced AM dissociation at respective ATP concentration. K2 = 4 s−1 and 10.2 s−1 at 1 and 5 μM ATP, respectively. For 1 μM ATP, N = 16, n = 987; for 5 μM ATP, N = 7, n = 500. For SolM-II at 10 μM ATP, N = 11, n = 520. N = Number of molecule, n = number of events. Important to note that for ensemble averaging, the AM attachment events with a lifetime of minimum 0.05 s or longer were selected. The method is described in detail in Veigel et al. (24) and Blackwell et al. (25)