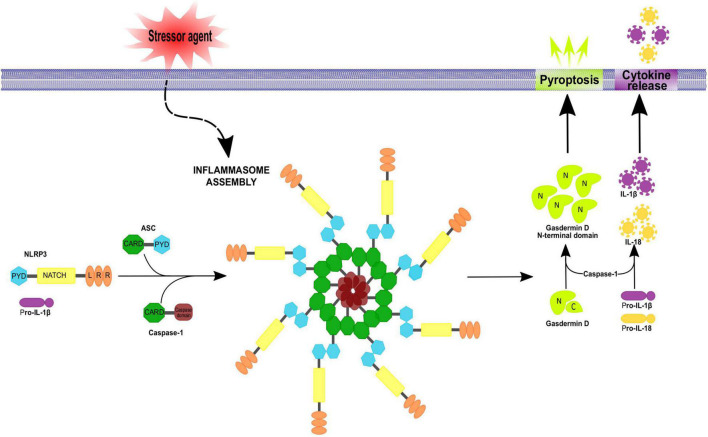
FIGURE 2.
Inflammasome assembly. Inflammasomes assemble in a stimulus-specific manner. Different stressor agents are able to induce inflammasome activation by NLRs or no-NLR inflammasome sensors proteins. Activation of the NLRP3 inflammasome involves ASC and procaspase-1 recruitment, resulting in ASC oligomerization into a macromolecular aggregate and subsequent activation of procaspase-1. Active caspase-1 then cleaves pro-IL-1β and pro-IL-18 to their mature forms IL-1 β and IL-18 which get secreted. In addition, caspase-1 can cleave gasdermin D, releasing its N-terminal fragment which translocate to the plasma membrane inducing pore formation and pyroptotic cell death. The apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) is an adaptor protein for many inflammasome complexes and is composed of CARD and PYD domains, the latter being necessary for homotypic interaction with a PYD-containing inflammasome sensor. Procaspase-1 features a CARD domain, in addition to its caspase domain, and homotypic CARD interactions result in direct or indirect (via ASC) recruitment of procaspase-1 to the inflammasome complex.