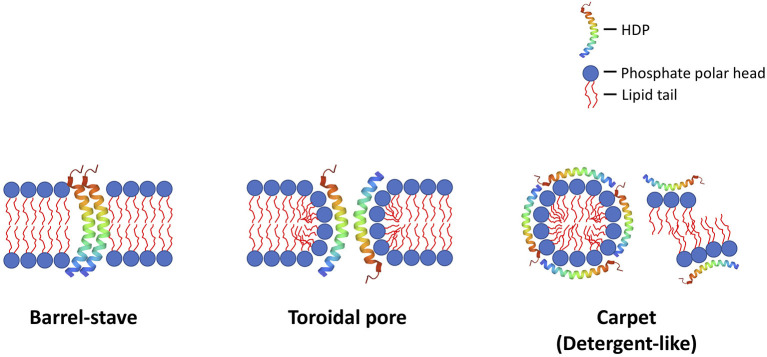
Figure 2.
Illustrations of the common membrane-permeabilizing mechanisms of host defense peptides (HDPs) against bacteria (and other microbes), namely: (1) barrel-stave; (2) toroidal pore; and (3) carpet (detergent-like) mechanisms. In the barrel-stave model, the HDPs act as a stave and penetrate vertically into the negatively charged, lipid bilayer bacterial membrane, creating permanent “barrel-shaped” pores. In the toroidal pore model, the HDPs interact with the negatively charged phosphate head groups electrostatically, distort the arrangement of the lipid bilayer, and create a transient membrane pore, with HDPs lining and stabilizing the internal part of the pore. In the carpet (detergent-like) model, the HDPs interact with the bacterial membrane electrostatically, and, upon reaching the critical concentration on the bacterial membrane, they result in membrane fragmentation / aggregation. These mechanisms result in destabilization of the membrane integrity, which leads to influx of fluid and efflux of intracellular content, culminating in cell lysis. Although less common (not shown in this figure), HDPs may also exert their antimicrobial action via binding to microbial intracellular targets (i.e., non-membrane-permeabilizing mechanisms), inhibiting DNA/RNA synthesis, protein synthesis and protein folding).