Abstract
Objective
The aim of the present study was to find a correlation of serum Suppression of tumorigenicity 2 (ST2) levels with severity of diastolic dysfunction on echocardiography and cardiac magnetic resonance imaging (CMRI) in heart failure with preserved ejection fraction (HFpEF) patients.
Methods
Fifty patients aged ≥18 years fulfilling diagnostic criteria for HFpEF were included. ST2 levels, 2D echocardiography and CMRI were performed. Left ventricular ejection fraction, E/A, Septal E/E’, left atrial volume index (LAVI), tricuspid regurgitation (TR), assessment of diastolic dysfunction, T1 mapping in milliseconds and late gadolinium enhancement (LGE) in percentage were noted. The primary outcome measure was to study correlation of ST2 levels with severity of diastolic dysfunction, whereas the secondary outcome measures were to study correlation of ST2 levels with native T1 mapping and LGE on CMRI.
Results
ST2 levels showed statistically significant and positive correlation with E/E’ (r = 0.837), peak TR velocity (r = 0.373), LAVI (r = 0.74), E/A (r = 0.420), and T1 values in milliseconds (r = 0.619). There was no statistically significant correlation between ST2 level and LGE in % (r = 0.145). The median ST2 levels in patients with E/E’ > 14 and E/E’ ≤ 14 were 110.8 and 36.1 respectively (p-value < 0.05). The mean ST2 levels were significantly higher in patients who had diastolic dysfunction grade III (126.4) and New York Heart Association class IV (133.3).
Conclusions
Evaluation of ST2 adds important information to support the diagnosis of left ventricular diastolic dysfunction in patients with HFpEF.
Keywords: Heart failure with a preserved ejection fraction (HFpEF), Cardiac magnetic resonance imaging (CMRI), Suppression of tumorigenicity 2 (ST2), Correlation, Late gadolinium enhancement (LGE)
1. Introduction
The global prevalence of heart failure (HF) is approaching epidemic proportions, as evidenced by the relentless increase in the number of HF hospitalizations and deaths. Patients with HF can be divided into (1) heart failure with reduced ejection fraction (HFrEF) (2) heart failure with mildly reduced ejection fraction (HFmrEF) and (3) heart failure with preserved ejection fraction (HFpEF).1 Patients with HFpEF have a devastating 5-year mortality rate (approaching 60.0%), costly morbidity (6-month hospitalization rate of 50.0%), and debilitating symptoms.2, 3, 4, 5, 6
Biomarkers are now routinely used to distinguish HF from other conditions, establish severity of the diagnosis and provide useful prognostic information in HF patients. Traditionally, Brain Natriuretic Peptide (BNP) and N-Terminal pro-Brain Natriuretic Peptide (NT pro-BNP) have been used as biomarkers. Recently other biomarkers to aid in the diagnosis, prognosis and management of HFpEF include soluble suppression of tumorigenicity 2 (sST2) and galectin 3. Some biomarkers are still under development e.g., Tissue Inhibitor of Metallo Proteinase-1 (TIMP-1).7
Echocardiography is the most widely used technique for the assessment and grading of the severity of diastolic dysfunction. Myocardial fibrosis is the one of the most significant factors in the development of diastolic dysfunction and plays a major role in the development of HFpEF, making it an attractive marker of disease progression and prognosis. There has been significant interest in determining whether cardiac magnetic resonance imaging (CMRI) parameters such as late gadolinium enhancement (LGE) - a marker of replacement fibrosis, and increased native myocardial T1 relaxation time - a marker of interstitial fibrosis, may prove to be meaningful for patients with HFpEF.
Most of the previous studies have demonstrated the use of serum biomarkers in patients of HFrEF. There is scarcity of data on biomarkers used in HFpEF. Previous studies in HFpEF patients have demonstrated a significant role of myocardial fibrosis as a pathogenetic mechanism. CMRI parameters are used as imaging markers for HFpEF. There are very limited previous studies which have correlated serum biomarker ST2 with imaging findings on echocardiography and CMRI. The aim of the present research was to find a correlation of serum ST2 levels with severity of diastolic dysfunction on echocardiography and CMRI in HFpEF patients.
2. Methods
This prospective observation study was conducted between April 2019 and April 2020 in the Department of Cardiology, in a tertiary care hospital, Pune, India. After approval from the institutional ethics committee, written informed consent was obtained from all the patients prior to enrolment explaining the risks and benefits of the procedure.
Patients >18 years of age fulfilling diagnostic criteria for HFpEF, i.e. clinical or radiological evidence of HF and left ventricular ejection fraction (LVEF) > 50% on transthoracic echocardiography were included. Patients who had chronic inflammatory conditions such as liver cirrhosis, other chronic liver diseases, autoimmune diseases, bronchial asthma, pulmonary fibrosis, chronic obstructive pulmonary disease, septic shock and estimated glomerular filtration rate <30 mL/min per 1.73 m2 were excluded.
A detailed history of all the patients was taken and physical examination was conducted. HF was defined as presence of two major criteria or one major and two minor criteria.8 The major criteria comprised paroxysmal nocturnal dyspnea, neck vein distention, rales, hepatojugular reflux, S3 gallop, central venous pressure >16 cm of water, circulation time of 25 s or longer, radiographic cardiomegaly and pulmonary edema, visceral congestion, or cardiomegaly. The minor criteria (accepted only if they cannot be attributed to another medical condition) were nocturnal cough, dyspnoea on ordinary exertion, pleural effusion, tachycardia (heart rate ≥120/minute), hepatomegaly and bilateral ankle edema.
ST2 levels were assessed by Sandwich monoclonal lateral flow immunoassay, on Aspect READER. A value ≥ 35 ng/mL is associated with worse prognosis in patients with HF and was used as a recommended cut-off for this study purpose.9 Haemogram, renal function tests, liver function tests along with ECG and chest X-ray were done on admission. Two dimensional echocardiography and colour Doppler was performed on Vivid E95 GE Healthcare System, Oslo, Norway. Echocardiography was performed on admission to assess LVEF and various parameters of diastolic function. E/A was noted in apical 4 chamber view using colour flow mapping and Pulsed Wave Doppler. Septal E/E′ was measured using Tissue Doppler Imaging. Left atrial volume was calculated using apical four- and two-chamber views 1–2 frames before mitral valve opening. Left atrial volume index (LAVI) was calculated by dividing left atrial volume by body surface area. Tricuspid regurgitation (TR) systolic jet velocity (m/sec) was calculated using parasternal and apical four-chamber view with color flow imaging to obtain highest Doppler velocity aligned with Continuous Wave Doppler. LVEF was estimated using modified Simpson's method. Assessment of diastolic dysfunction using various parameters and grading was done as follows- Normal, Grade 1, Grade II and Grade III.10
CMRI was performed using a 3 T system (Ingenia, Philips Healthcare, Amsterdam, Netherlands) according to standard protocol within 15 days of admission. T1 mapping in milliseconds and LGE in percentage was noted. T1 mapping was done using the Shortened Modified Look-Locker Inversion Recovery sequence in which Inversion Recovery pulse over multiple heart beats were used. LGE was evaluated 10 min after administration of a bolus of gadolinium based contrast agent (0.1–0.2 mmoL/Kg) to identify regional myocardial fibrosis using the Phase-Sensitive Inversion Recovery technique. LGE imaging was performed using standard long-axis views of the left ventricle and a contiguous short-axis stack from the mitral valve annulus to the left ventricular apex. Regional fibrosis was identified by LGE within the myocardium, defined quantitatively by myocardial postcontrast signal intensity two standard deviations above that within a reference region of remote myocardium within the same slice. The extent of LGE was calculated after manual tracing of endocardial and epicardial borders on each short-axis slice. LGE volume was expressed as a percentage of total myocardial mass.
The primary objective was to study correlation of ST2 levels with the severity of diastolic dysfunction, whereas the secondary objectives were to study correlation of ST2 levels with native T1 mapping and LGE on CMRI. The sample size was calculated by a formula N = [(Zα+Zβ)/C]2 + 3 where C = 0.5 ∗ ln [(1 + r)/(1-r)].11 We have taken Zα a standard normal variate at 1% type 1 error (p < 0.01) as 2.58 and Zβ the standard normal deviate for β power 90% type II error as 1.28. Farcas AD et al reported a correlation of 0.547.12 The total sample size of 42 was calculated by above method. To validate the results 50 patients were included in the study.
The analysis of data was done using Statistical Package for Social Sciences, Version 20.0 from IBM Corporation, Armonk, NY, USA. The data on categorical variables are shown as n (% of cases). The parametric data of continuous variables are presented as mean and standard deviation (SD), whereas non-parametric data are presented as median. Comparison of the distribution of categorical variables was done using Fisher's exact test. Comparison of non-parametric continuous variables was done using the Mann–Whitney U test. The Pearson's correlation coefficient was used to find the correlation between E/A, E/E′, LAVI, Peak TR velocity, Native T1 mapping and LGE with ST2 values. Analysis of variance (ANOVA) test was used to compare the mean ST2 value with New York heart association (NYHA) classification and grade of diastolic dysfunction. The confidence limit for significance was fixed at 95% level with a p-value < 0.05.
3. Results
The mean ± SD of the age of the study population was 64.8 ± 10.6 years. The median ST2 level was 42.95 U/mL (IQR 29.6–83.7). The clinical and demographic profile of the study population is summarized in Table 1.
Table 1.
Clinical and demographic profile of the study population.
| Clinical and demographic profile | Number | % |
|---|---|---|
| Age group in years | ||
| ≤50 | 6 | 12 |
| 51–60 | 4 | 8 |
| 61–70 | 19 | 38 |
| 71–80 | 13 | 26 |
| >80 | 8 | 16 |
| Gender | ||
| Male | 26 | 52 |
| Female | 24 | 48 |
| NYHA functional class | ||
| I | 8 | 16 |
| II | 18 | 36 |
| III | 19 | 38 |
| IV | 5 | 10 |
| Diabetes mellitus | 30 | 60 |
| Hypertension | 34 | 68 |
| Dyslipidaemia | 24 | 48 |
| ST2 levels U/mL | ||
| <35 | 18 | 36 |
| ≥35 | 32 | 64 |
| Diastolic dysfunction grades | ||
| I | 23 | 46 |
| II | 20 | 40 |
| III | 7 | 14 |
NYHA- New York Heart Association.
The median ST2 levels in patients with E/E’ > 14 and E/E’ ≤ 14 were 110.8 and 36.1 respectively (p-value < 0.05). The mean ST2 level was significantly higher in patients who had diastolic dysfunction grade III (126.4 ± 16.6) as compared to grades I (31.1 ± 8.0) and II (69.0 ± 27.6). The mean ST2 level was significantly higher in patients who had NYHA class III (81.7 ± 22.2) and IV (133.3 ± 17.0) as compared to NYHA class I (27.1 ± 3.4) and class II (32.4 ± 8.3). There was a statistically significant difference between ST2 levels and grade of diastolic dysfunction. The higher percentage of patients who had diastolic dysfunction grade III had ST2 levels >35 U/mL (Table 2).
Table 2.
Grades of diastolic dysfunction according to ST2 values.
| Grade of diastolic dysfunction | ST2 level (U/mL) |
Total n (%) | p-value | |
|---|---|---|---|---|
| >35 n (%) | ≤35 n (%) | |||
| I | 14 (60.9) | 9 (39.1) | 23 (100.0) | 0.049 |
| II | 17 (85.0) | 3 (15.0) | 20 (100.0) | |
| III | 7 (100.0) | 0 (0.0) | 7 (100.0) | |
| Total | 38 | 12 | 50 (100.0) | |
Fisher's exact test was used.
ST2- Suppression of tumorigenicity 2.
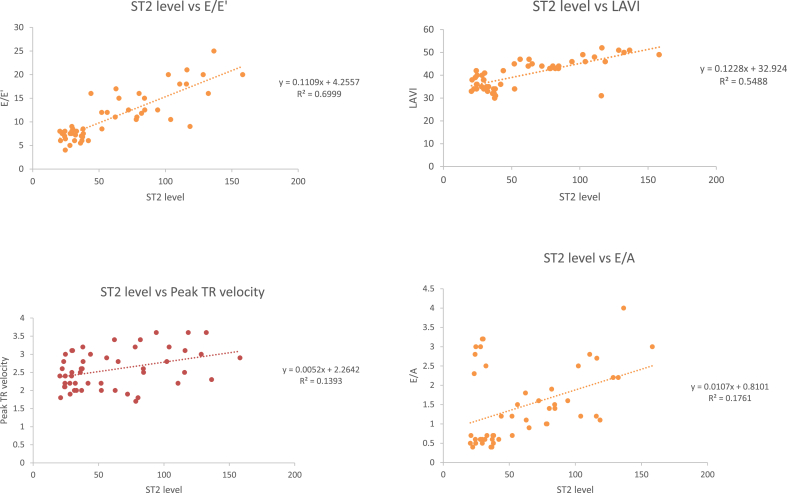
ST2 levels showed statistically significant (p-value < 0.001) and positive correlation (r = 0.836) with E/E′, peak TR velocity (p-value = 0.007, r = 0.373), LAVI (p-value < 0.001, r = 0.74) and E/A (p-value = 0.002, r = 0.42). As ST2 levels increased, E/E’, peak TR velocity, LAVI and E/A also increased (Fig. 1-A, B, C, D).
Fig. 1.
A: Correlation of ST2 Levels with E/E′, B: Correlation of ST2 levels with peak TR velocity, C: Correlation of ST2 levels with left atrial volume index, D: Correlation of ST2 levels with E/A.
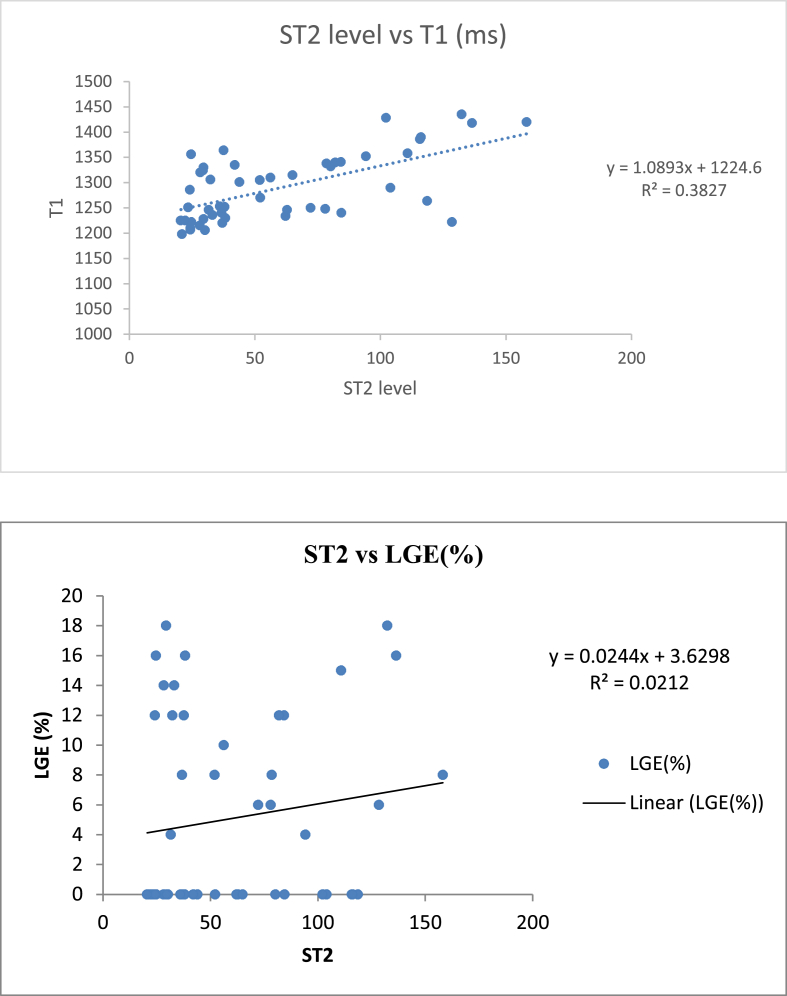
ST2 levels showed statistically significant (p-value < 0.001) and positive correlation (r = 0.619) with T1 values (in milliseconds). As ST2 levels increased, T1 values (in milliseconds) also increased (Fig. 2-A). There was no statistically significant (p-value = 0.125) correlation (r = 0.145) between ST2 level and LGE in % (Fig. 2-B).
Fig. 2.
A: Correlation of ST2 levels with native T1 values, B: Correlation of ST2 levels with LGE.
4. Discussion
In the present study, the correlation of a novel biomarker serum ST2 in patients of HFpEF was evaluated. In the present study, ST2 levels showed statistically significant and positive correlation with E/E’, peak TR velocity, LAVI, E/A, and T1 values (in milliseconds). There was no statistically significant correlation between ST2 level and LGE in %.
In the present study, the median ST2 level was 42.95 U/mL. Shah KB et al13 reported that the median ST2 value was 38.4 U/mL in patients with HFpEF. In the study by Januzzi JL et al on ST2 and prognosis in acutely decompensated heart failure, it was concluded that the threshold of 35 U/mL provides a reasonable reference limit above which risk for adverse outcome rises linearly with the degree of elevation.9 In the present study, 32 (64.0%) and 18 (36.0%). patients had ST2 levels ≥35 U/mL and <35 U/mL respectively. AbouEzzeddine OF et al14 and Najjar E et al15 reported that patients with higher ST2 levels had higher NYHA functional class in patients with HFpEF (p-values = 0.029, and 0.017 respectively). Our research substantiated the findings of these studies.
Farcas AD et al12 reported that ST2 levels were 30.67 ± 21.06, and 43.54 ± 17.49 in patients with diastolic dysfunction grades I and II respectively (p-value = 0.016). It was further stated that ST2 levels increased proportionally with the presence and worsening of the diastolic dysfunction. In the present study, mean ± SD of ST2 values were 31.1 ± 8.0, 69.0 ± 27.6, and 126.4 ± 16.6 in patients with diastolic dysfunctions grades I, II and III respectively. In the present study, a higher percentage of patients who had diastolic dysfunction grade III had ST2 levels >35 U/mL. Januzzi JL et al reported that ST2 levels above the cut off value of 35 U/mL were associated with a poorer prognosis.9
In the present study, there was a significantly positive correlation (r = 0.420) between ST2 levels and E/A which is a marker of diastolic dysfunction. Farcas AD et al12 and Ojji DB et al16 also reported positive correlation of 0.22 and 0.43 respectively between ST2 levels and E/A. In the present study, there was a significantly positive correlation (r = 0.837) between ST2 levels and E/E′ which is a marker of diastolic dysfunction. Shah KB et al13 and Farcas AD et al12 also reported a significantly positive correlation of 0.210 and 0.215 respectively between ST2 levels and E/E’. However, in the study by Sugano A et al there was not a significant association between ST2 and E/E’ (p-value = 0.57).17 E/E′ is a good surrogate noninvasive marker to denote raised left ventricular filling pressures and the recommended cut off value is 14.18 In the study by Shah KB et al, cut off value of 15 was used for E/E’.13 Shah KB et al, reported that the median ST2 level in HFpEF patients with E/E’ > 15 and E/E’ < 15 was 58.7 (IQR 50.1–144.4) U/mL and 29.6 (IQR 23.2–50.6) U/mL respectively (p-value = 0.002).13 In the present study, the median ST2 level in patients with E/E’ > 14 and E/E’ ≤ 14 was 110.8 U/mL and 36.1 U/mL respectively (p-value < 0.05).
In the present study, there was a significantly positive correlation (r = 0.373) between ST2 levels and peak TR velocity which is a marker of diastolic dysfunction. Sugano A et al reported a weak positive correlation (r = 0.180) between ST2 and TR velocity with p-value 0.0019.17 In the present study, there was a significantly positive correlation (r = 0.74) between ST2 levels and LAVI which is a marker of diastolic dysfunction. Najjar E et al reported a significantly positive correlation (r = 0.276) between ST2 levels and LAVI (p-value = 0.019).15 Zile MR et al in the PARAMOUNT study observed a significant correlation (r = 0.25) between ST2 biomarker and LAVI with p-value <0.001.19 However, in the study by Sugano A et al there was no significant association between ST2 and LAVI (p-value = 0.96).17
T1 mapping is an imaging marker of myocardial fibrosis as observed in multiple previous studies.20, 21, 22, 23 There has been no previous study till date which has assessed the relation between ST2 and native T1 mapping in patients of HFpEF. Park YH et al conducted a study on correlation of soluble ST2 and left ventricular mass index with native T1 mapping value in patients with dilated cardiomyopathy. It was stated that the sST2 and mass index showed a moderate linear correlation with native T1 value (p = 0.012, R2 = 0.382).24 In the present study, there was a significant positive correlation (r = 0.619) between ST2 levels and T1 mapping (in milliseconds) on CMRI. In the present study, there was no significant correlation (r = 0.145) between ST2 and LGE on CMRI. Similarly, in the study by Quick S et al the correlation (r = - 0.13) between ST2 values and LGE was 0.130 (p-value = 0.32).25
The major strength of the present research is that there is no previous study reported till date which has assessed the relation between ST2 and native T1 mapping in patients of HFpEF. Potential limitations of the study merit consideration. The study was mainly limited by the small sample size and the single centre design. As a consequence, these findings should be considered hypothesis-generating and needs to be confirmed in larger populations. This study was done in only HFpEF patients and no control group was taken. NT-proBNP/BNP level measurements were not done. Multicentric studies with a large sample size should be undertaken to substantiate the research findings described in this paper.
5. Conclusions
ST2 levels showed statistically significant and positive correlation with E/E′, peak TR velocity, LAVI, E/A, and T1 values (in milliseconds). There was no statistically significant correlation between ST2 level and LGE in %. The median ST2 level in patients with E/E’ > 14 was significantly higher as compared to patients with E/E’ ≤ 14. The mean ST2 level was significantly higher in patients who had diastolic dysfunction grade III and NYHA class IV. Evaluation of ST2 adds important information to support the diagnosis of LV diastolic dysfunction and can be a good predictor of diastolic dysfunction in patients with HFpEF.
6. What is already known?
Echocardiography is widely used for assessment of diastolic function. There are studies which have compared serum ST2 levels with clinical and echocardiographic parameters in HFpEF.
7. What does this study add?
CMRI is the gold standard for assessing myocardial fibrosis for which parameters like T1 mapping and LGE are useful. There are very limited studies that have correlated serum ST2 levels with CMRI in patients of HFpEF. There is no previous study reported till date which has assessed the correlation between ST2 and native T1 mapping and LGE in % in patients of HFpEF. In the present research, correlation between ST2 and T1 mapping and LGE in % was studied.
Declaration of competing interest
Dr. Vivek Agrawal, Dr. Suhas Hardas, Dr. Hasmukh Gujar and Dr. Deepak Phalgune declare that they have no conflict of interest. The manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work.
Contributor Information
Vivek Agrawal, Email: vivekmaheshagrawal@gmail.com.
Suhas Hardas, Email: suhas_h@hotmail.com.
Hasmukh Gujar, Email: drhasmukhgujar@gmail.com.
Deepak S. Phalgune, Email: dphalgune@gmail.com.
References
- 1.McDonagh T.A., Metra T., Adamo M., et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42:3599–3726. [Google Scholar]
- 2.Little W.C., Zile M.R., Klein A., et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383–385. doi: 10.1016/j.amjcard.2006.01.106. [DOI] [PubMed] [Google Scholar]
- 3.Rector T.S., Carson P.E., Anand I.S., et al. Assessment of long-term effects of irbesartan on heart failure with preserved ejection fraction as measured by the Minnesota Living with Heart Failure Questionnaire in the I-Preserve Trial. Circ Heart Fail. 2012;5:217–225. doi: 10.1161/CIRCHEARTFAILURE.111.964221. [DOI] [PubMed] [Google Scholar]
- 4.Zile M.R., Kjellstrom B., Bennett T., et al. Effects of exercise on left ventricular systolic and diastolic properties in patients with heart failure and a preserved ejection fraction versus heart failure and a reduced ejection fraction. Circ Heart Fail. 2013;6:508–516. doi: 10.1161/CIRCHEARTFAILURE.112.000216. [DOI] [PubMed] [Google Scholar]
- 5.Zile M.R., Bourge R.C., Redfield M.M., et al. Randomized, double- blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2014;2:123–130. doi: 10.1016/j.jchf.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Carson P.E., Anand I.S., Win S., et al. The hospitalization burden and post-hospitalization mortality risk in heart failure with preserved ejection fraction: results from the I-PRESERVE trial (Irbesartan in Heart Failure and Preserved Ejection Fraction) JACC Heart Fail. 2015;3:429–441. doi: 10.1016/j.jchf.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Chow S.L., Maisel A.S., Anand I., et al. Role of biomarkers for the prevention, assessment, and management of heart failure. a consensus statement for healthcare professions from the American Heart Association. Circulation. 2017;135:e1054–e1091. doi: 10.1161/CIR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 8.Ho K.K., Pinsky J.L., Kannel W.B., et al. The epidemiology of heart failure:the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 9.Januzzi J.L., Mebazza A., Somma D.S. ST2 and prognosis in acutely decompensated heart failure: the International ST2 Consensus Panel. Am J Cardiol. 2015;115:26B–31B. doi: 10.1016/j.amjcard.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Hulley S.B., Cummings S.R., Browner W.S., et al. 4th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. Designing Clinical Research: An Epidemiologic Approach; p. 79. Appendix 6C. [Google Scholar]
- 12.Farcaş A.D., Anton F.P., Goidescu C.M., et al. Serum soluble ST2 and diastolic dysfunction in hypertensive patients. Dis Markers. 2017;2017:2714095. doi: 10.1155/2017/2714095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah K.B., Kop W.J., Christenson R.H., et al. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem. 2011;57:874–882. doi: 10.1373/clinchem.2010.159277. [DOI] [PubMed] [Google Scholar]
- 14.AbouEzzeddine O.F., McKie P.M., Dunlay S.M., et al. Suppression of tumorigenicity 2 in heart failure with preserved ejection fraction. J Am Heart Assoc. 2017 Feb 18;6(2) doi: 10.1161/JAHA.116.004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najjar E., Faxén U.L., Hage C., et al. ST2 in heart failure with preserved and reduced ejection fraction. Scand Cardiovasc J. 2019;53:21–27. doi: 10.1080/14017431.2019.1583363. [DOI] [PubMed] [Google Scholar]
- 16.Ojji D.B., Opie L.H., Lecour S., et al. The effect of left ventricular remodelling on soluble ST2 in a cohort of hypertensive subjects. J Hum Hypertens. 2014;28:432–437. doi: 10.1038/jhh.2013.130. [DOI] [PubMed] [Google Scholar]
- 17.Sugano A., Seo Y., Ishizu T., et al. Soluble ST2 and brain natriuretic peptide predict different mode of death in patients with heart failure and preserved ejection fraction. J Cardiol. 2019;73:326–332. doi: 10.1016/j.jjcc.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur J Echocardiogr. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 19.Zile M.R., Jhund P.S., Baicu C.F., et al. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejecti-on fraction: data from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction study. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellims A.H., Shaw J.A., Stub D., et al. Diffuse myocardial fibrosis evaluated by post-contrast T1 mapping correlates with left ventricular stiffness. J Am Coll Cardiol. 2014;63:1112–1118. doi: 10.1016/j.jacc.2013.10.084. [DOI] [PubMed] [Google Scholar]
- 21.Duca F., Kammerlander A.A., Tufaro C.Z., et al. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imag. 2016;9 doi: 10.1161/CIRCIMAGING.116.005277. [DOI] [PubMed] [Google Scholar]
- 22.Mascherbauer J., Marzluf B.A., Tufaro C., et al. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imag. 2013;6:1056–1065. doi: 10.1161/CIRCIMAGING.113.000633. [DOI] [PubMed] [Google Scholar]
- 23.Sado D.M., Flett A.S., Banypersad S.M., et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–1441. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]
- 24.Park YH, Lee SY, Jung SM, et al. Soluble ST2/LVMI correlates with native T1 mapping value in 3T cardiac magnetic resonance in patients with dilated cardiomyopathy. European Society of Cardiology. Heart Failure 2019 -6th World Congress on Acute Heart Failure 25-28 May 2019, Athens, Greece P-958.
- 25.Quick S., Waessnig N.K., Kandler N., et al. Soluble ST2 and myocardial fibrosis in 3T cardiac magnetic resonance. Scand Cardiovasc J. 2015;49:361–366. doi: 10.3109/14017431.2015.1076936. [DOI] [PubMed] [Google Scholar]