Abstract

Perylene derivatives constitute a promising class of compounds with technological applications mainly due to their optoelectronic properties. One mechanism proposed to synthesize them, starting from binaphthyl derivatives, is anionic cyclodehydrogenation (under reductive conditions). However, the scope of this reaction is limited. In the present study, we report a theoretical and experimental analysis of this particular reaction mechanism for its use in the synthesis of 1-substituted perylenes. Different substituents at position 2 of 1,1′-binaphthalene were evaluated: −OCH3, −OSi(CH3)2C(CH3)3, and −N(CH3)2. Based on density functional theory (DFT) calculations on the proposed mechanism, we suggest that the cyclization takes place from binaphthyl dianion instead of its radical anion. This dianion has an open-shell diradical nature, and this could be the species that was detected by EPR in previous studies. The O-substituted derivatives could not afford the perylene derivatives since their radical anions fragment and the necessary binaphthyl dianion could not be formed. On the other hand, 49% of N,N-dimethylperylen-1-amine was obtained starting from the N-substituted 2-binapthyl derivative as a substrate, employing a simpler experimental methodology.
Introduction
Two hot topics involve new organic compounds with high impact, organic light-emitting diodes (OLEDs) and organic solar cells (OSCs) in photovoltaic devices.1 At present, OLEDs are employed in the screen of smart devices and in panels for lighting applications and represent an eco-friendly alternative to the traditional systems.2 One of the challenges in this field is the generation of efficient pure light emission from the diode with a narrow line. Such an emitter is essential to complete the spectrum for a full-color display based on the principle of additive mixing.3 In addition, it is expected that OSCs could be massively employed within a few years because of their properties. They are potentially flexible, semi-transparent, and lightweight, and their fabrication might be implemented with available and low-cost technologies using eco-friendly materials.1a One of the main goals in this field is to increase the power conversion efficiencies. Historically, OSCs were built with fullerene-based acceptors. However, recent developments propose that other polycyclic compounds could replace fullerene in the next generation of high-performance OSCs.4 For these reasons, the search for new industrial processes and molecular systems for these applications is continuously growing.1a
In general, rylenes, perylene included, constitute an important class of compounds that show appropriate optoelectronic properties, fundamental for their application in both OLED and OSC devices.5 Perylene shows characteristic fluorescence with high quantum yield, which varies depending on both the nature of the substituents attached to the polycycle and their positions (e.g., the axial or equatorial regions). Perylene bisimide (PBI) and perylene monoimide are two of the most studied compounds within the rylene family, mainly because of their photophysical properties.
Different strategies have been developed for the synthesis of perylene: condensation of quinone derivatives,6 base-induced dimerization of benzoisoquinolinediones,7 Cu- or Pd-catalyzed annulation reactions,8 cyclodehydrogenation of 1,1′-binaphthyl derivatives (e.g., Scholl reaction),9 and decarboxylation of the perylene-3,4,9,10-tetracarboxylic dianhydride under a high temperature and pressure.10 These strategies are mainly focused on the formation of the perylene ring; however, only two synthetic ways, reported until now, are useful to obtain bay-substituted perylene by condensation of the substitute quinolones6 and Scholl reaction of a 1,1′-binaphthyl derivative.9a,9e Furthermore, most of the reported examples of substituted perylene in the different positions,11bay,12ortho,13 or peri,14 discuss the incorporation of functional groups to an already formed perylene nucleus.
In 1968, Solodovnikov et al. published the synthesis of perylene from 1,1′-binaphthalene (1a) in 1,2-dimethoxyethane with an excess of potassium metal under vacuum at room temperature.15 More recently, Rickhaus et al. optimized this methodology,9b which involves reductive induced cyclization of 1,1′-binaphthalene by the action of three or more equivalents of potassium metal in hot tetrahydrofuran with a quantitative yield (Scheme 1). This anionic cyclodehydrogenation reaction was also employed for the synthesis of 1-azaperylene,9c 13,13′-dibenzo[b]perylenyl derivatives,16 and heteroaromatic polycyclic compounds, in some cases using a mechanochemical methodology, among others.17 However, this methodology was not employed for the synthesis of 1-substituted perylene derivatives.
Scheme 1. Anionic Cyclodehydrogenation Reaction for the Synthesis of Perylene.
Adapted with permission from ref (9b).
A deeper knowledge of the involved mechanism is essential to extend the scope of the anionic cyclodehydrogenation reaction for the synthesis of bay-substituted perylene derivatives. According to this, we carried out a combined experimental and molecular modeling analysis using a selected 2-substituted-1,1′-binaphthalene (Figure 1) to evaluate the application of this reaction mechanism for the preparation of 1-substituted perylenes.
Figure 1.

2-Substituted-1,1′-binaphthalenes employed as substrates in the anionic cyclodehydrogenation reactions.
Results and Discussion
Reactivity of 1,1′-Binaphthalene
The first substrate for the anionic cyclodehydrogenation reactions, 1,1′-binaphthalene (1a), was synthesized following a Suzuki–Miyaura coupling reaction of 1-bromonaphthalene and 1-naphtylboronic acid catalyzed by Pd(0).18
It is important to mention that the original reaction conditions for the synthesis of perylene, proposed in ref (9b), were slightly modified by changing the solvent from tetrahydrofuran (THF) to toluene (Scheme 1) to replace the use of pressure vessels by simpler Schlenk tube flasks.19 Within the new reaction conditions, similar yields of perylene (2a) were obtained (90%), starting from 1,1′-binaphthalene (1a), compared with the originally reported yields (higher than 90%).9b This result validates the use of the new reaction conditions.
Rickhaus et al. proposed a reaction mechanism based on previous research and the experimental observations are as follows: the presence of radical species detected by electron paramagnetic resonance (EPR) and the formation of H2 bubbles observed at the end of the experiments.9b,15 There are two reaction pathways (Scheme 2, paths A and B) by which 1a could be converted into perylene requiring two single-electron reductions, cyclization with C–C bond formation, and two C–H bond-breaking steps to give 2a. The initial anion radical 1a•– can undergo a cyclization to form 3a•– (path A), which takes the second electron to give 3a2–; or take a second electron to give the dianion 1a2– which subsequently gives 3a2– (path B); the latter is called the pivotal intermediate for the formation of perylene by Rickhaus et al.
Scheme 2. Proposed Mechanism for the Anionic Cyclodehydrogenation Reaction.
Adapted with permission from ref (9b).
In this study, we carry out the first molecular modeling studies based on the density functional theory (DFT) calculations of the proposed mechanism for the anionic cyclodehydrogenation reaction (Scheme 2). The hybrid-GGA functionals B3LYP20 and ω-B97XD21 were employed. All calculations reported here correspond to the B3LYP/6-31+G(d) level of theory, whereas the results with ω-B97XD are included in the Supporting Information (SI). In general, similar tendencies were obtained with both functionals. The polarizable continuum model (IEF-PCM)22 was employed for modeling the solvent used in the reactions (toluene, ϵ = 2.37).
Different electronic states of the dianion 1a2– were modeled concluding that this intermediate has the character of an open-shell (OS) singlet ground state,23 being a diradical dianion (DAOS). The spin density distribution shows the same contribution at the two naphthyl rings (Scheme 3, Figure S1, and Tables S1 and S3). Based on these new findings, either 1a•– or 1a2– could be the intermediary species detected by EPR instead of just 1a•– as was previously thought.15
Scheme 3. Profile of Energy for the Ring Closure Reaction of Substrate 1a.

The color red is employed to remark path A and blue for path B of Scheme 2. The plots of the total spin density of radical anion (1a•–) and open-shell dianion (1a2–) and the corresponding transition states were added as an inset. All free energy values are expressed in kcal/mol and adjusted at 363 K.
After the electron transfer from K to 1a to form 1a•– and/or 1a2–, the next reaction step is the ring closure (path A or B, Scheme 2). Two possibilities were evaluated (Table S3), either from 1a•– or from 1a2–. The energy profile for these reactions is presented in Scheme 3.
In the first place, it should be noted that the barrier (ΔG‡) for the ring closure from the DAOS1a2– is lower than that from 1a•–, 26.1 and 32.3 kcal/mol, respectively.24 In addition, ΔGR is also in favor of the ring closure from 1a2– instead of 1a•– (7.1 vs 30.4 kcal/mol, respectively). We propose that the main differences are related to electronic rather than geometrical/sterical reasons. In the case of OS 1a2–, after coupling, a closed-shell intermediary 3a2– was obtained. The diradical character of 1a2– favors a radical-radical intramolecular recombination reaction,25 as shown by the total spin density at the transition state (see Figure S2). On the other hand, after the coupling of 1a•–, the product 3a•– still presented an unpaired electron.
Finally, the next steps for the formation of perylene include the elimination of two H atoms or an H2 molecule as revealed by the bubble formation in the last stages of the experimental reactions. The participation of H• within the very reductive reaction conditions is not clear at all; hence, DFT studies of that part of the reaction were not carried out.17c
After the molecular modeling analysis of the proposed mechanism, evidence in favor of the participation of the diradical dianion 1a2– (barely proposed by Rickhaus et al.9b) as the key intermediary for the ring closure was found. In order to confirm this hypothesis, different 2-substituted-1,1′-binaphthalenes were selected for the experimental studies that were divided into two groups: 2-(O-substituted)-1,1′-binaphthyl derivatives, 1b and 1c, in one group and 2-(N-substituted)-1,1′-binaphthalene (1d) in the other group (see Figure 1).
Reactivity of 2-(O-Substituted)-1,1′-binaphthalene
The reactions of radical anions of aromatic ethers have been studied by various authors.26 Azzena et al.27 reported that anisole reacts with potassium in THF to exclusively yield phenol by demethylation through fragmentation of the PhO–CH3 bond, regardless of the temperature. On the other hand, in solvents with a very low dielectric constant (e.g., aliphatic hydrocarbons, toluene, and dioxane), demethoxylation was the main reaction pathway, by breaking of the Ph–OCH3 bond, leading to benzene as the product.
The 1,1′-binaphthyl derivatives 1b (2-methoxy-1,1′-binaphthalene) and 1c ([1,1′-binaphthalen]-2-yloxy)(tert-butyl)dimethylsilane) were synthesized in two simple steps. A photoinduced nucleophilic substitution reaction between 1-iodonaphthalene and 2-napthol was followed by methylation with (CH3)2SO4 to give 1b, in an overall isolated yield of 50%, or followed by silyl protection with tert-butylchlorodimethylsilane leading to 1c.28,29
The anionic cyclodehydrogenation reactions of 1b and 1c were carried out. The results are shown in Scheme 4.
Scheme 4. Anionic Cyclodehydrogenation Reactions of 1b and 1c.
Yields are expressed in relative areas obtained by GC.
When the reaction of substrate 1b was carried out, perylene (2a) was obtained instead of the substituted 1-methoxy-perylene at 38% relative yield. On replacing the methyl group by −Si(CH3)2C(CH3)3, a slightly higher yield of 2a was obtained (43%), and 39% of product 4, but none of the 1-substituted perylene, was observed. The anionic cyclodehydrogenation reaction with 4 as a substrate failed too.30
The results are consistent with the participation of radical anions as intermediates in the reactions. In the case of 1b, fragmentation of the aryl–OCH3 bond was the main reaction; meanwhile, by replacing the −CH3 group by a better leaving group −Si(CH3)2C(CH3)3, the aryl O–SiR fragmentation was also observed (Scheme 4).
Reactivity of 2-(N-Substituted)-1,1′-binaphthalene
As in the case of alkyl aryl ethers, there are also a few examples studying the reactivity of radical anions of N,N-dimethylanilines.31 In one of these cases, the reactivity of 4-methoxy-N,N-dimethylaniline under K in THF was studied. It is important to mention that no reactions over the dimethylamino group were observed; moreover, demethylation of the methoxy group and demethoxylation are the reactions observed. The loss of the methoxy group was the main reaction pathway of the radical anions generated in isooctane, a solvent with a polarity similar to toluene (ϵ = 1.94 and ϵ = 2.37, respectively).31a In another example, reactions of N,N-dimethylanilines in THF by using 2 equiv of Li as a reductive reagent were studied.31b Products derived from the aryl-N(CH3)2 bond cleavage were observed. However, when K was employed, a complex reaction mixture was found with no evidence of aryl–N(CH3)2 bond cleavage.
In contrary to the results obtained with substrates 1b and 1c, the radical anion of N-substituted 2-binaphthyl derivative 1d did not undergo fragmentation; hence 1d2–, could be formed to finally give 3d2– after a couple of reaction steps (see Scheme 2).
As in the case of 1b, N,N-dimethyl-[1,1′-binaphthalen]-2-amine (1d) was synthesized in two simple steps: a photoinduced substitution reaction of 1-iodonaphthalene and 2-naphthylamine in NH3(l)32 followed by methylation with (CH3)2SO4 to give 55% of 1d.9a
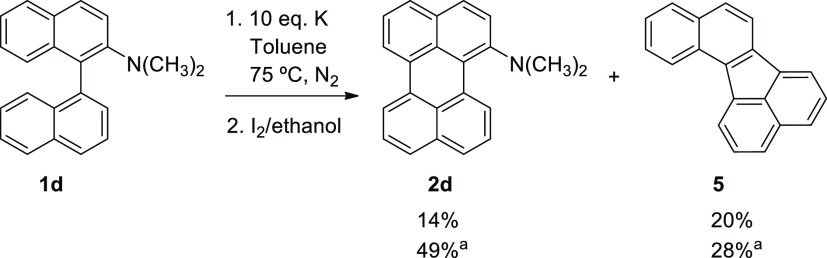
The anionic cyclodehydrogenation reaction of 1d as a substrate was carried out and 2d was obtained (Scheme 5).
Scheme 5. Anionic Cyclodehydrogenation Reaction of 1d.
Percentages are isolated yields. The oxidant agent used in the second step is O2.
N,N-Dimethylperylen-1-amine (2d) was obtained in a 14% isolated yield. Traces of perylene were also detected in all reactions (<4%). The yield of 2d was improved on changing the workup process by exposing the reaction mixture to O2 overnight, from 14 to 49%.17c However, a perylene isomer, benzo[j]fluoranthene (5), was the main byproduct in all our reactions. A similar behavior was observed in the pyrolysis of 1,1′-binaphthalene (1a) with hexanes as a radical source at 1100 °C. Under these conditions, the cyclodehydrogenation of 1a forms perylene and the five-membered ring product 5.33 For improving the yield of the desired perylene derivative (2d), different reaction conditions were assessed by changing the temperature and the equivalents of K (Table S6).
According to these references and our experimental results, the fragmentation, aryl–N(CH3)2 or aryl N–(CH3)2, of the radical anion of 1d is not the main reaction pathway followed by this intermediary (since binaphthalene or any demethylated compound was not found). Moreover, experimentally, a wine-red color was also observed in the reaction mixture as it was proposed before for the perylene dianion intermediate.15 It should be noted that this color was not observed in reactions of 1b and 1c. Since 1d•– did not fragment, we propose that 1d2– was formed after an electron transfer from the metal to this radical anion. Then, 1d2– would lead the substituted perylene 2d after the reaction workup. The ring closure from 1d•– to 3d•– and formation of 3d2– after a second electron transfer could be discarded based on the DFT analysis of the mechanism (Table S4).
The major byproduct observed is benzo[j]fluoranthene (5), a compound without the dimethylamine group; it must proceed from an intermediary not found in the reactions with 1b and 1c. We propose that benzo[j]fluoranthene comes from fragmentation of the dianion 1d2–. According to the DFT calculations, this fragmentation is not energetically favored over ring closure of 1d2– to give 3d2– (Table S5). However, there would be a competition between an irreversible reaction (cleavage of the aryl–N(CH3)2 bond) and an equilibrium reaction (1d2– ⇆ 3d2–); thus, relative yields between 2d and 5 could vary according to the changes in the experimental conditions of the reactions (Table S6).
Finally, to summarize the information about the different analyzed reactions, an extended reaction scheme is shown (Scheme 6) including the possible pathways involved in the formation of the different observed products.
Scheme 6. Proposed Extended Mechanisms Involved in the Anionic Cyclodehydrogenation Reactions Carried out for Substrates 1a–d.

In this mechanism, the reactions of the radical anion (in red) differ from the reactions of the dianion (in blue). In the case of the radical anion, the fragmentations at both the O–aryl and O–alkyl levels are important and there is no formation of the dianion necessary for the cyclization indeed. The reactivity or instability of aryl–O–alkyl rules out the application of this anionic cyclodehydrogenation reaction for the synthesis of bay O-substituted perylenes. This scenario would even hold if diaryl ethers were used or if O is replaced by other heteroatoms from group 6, such as S or Se, whose anion radicals are even more fragile.34 If the radical anion is long lived enough to receive a second electron forming a dianion, the chances to form the dihydroperylene derivative increase as it was experimentally found in the case of the N-substituted substrate (1d), with the formation of fluoranthene derivative as an undesired side reaction.
Conclusions
Based on the presented results and analyzing the possible reactions of the formed intermediates, radical anions, and dianions, the reactivity of substrates 1b–d toward the anionic cyclodehydrogenation reaction is explained. Using a simpler reaction condition and toluene as a solvent, 1-N(CH3)2 perylene and perylene were obtained in 49 and 90% isolated yields, respectively. We demonstrated the synthetic limitations of this mechanism and could confirm experimentally and with computational modeling that the dianion intermediate 12– is necessary as a precursor for perylene formation. Thus, we were able to determine the possibility of an extension of the reaction scope of anionic cyclodehydrogenation to the synthesis of substituted perylene derivatives in the bay position with −N(CH3)2 as a substituent and not with alkoxy groups due to fragmentation of their radical anions. This methodology could work for 2-C-substituted binaphthalenes in which their radical anion will be stable34c and after the second electron accepting the anionic cyclodehydrogenation could occur.25 This last hypothesis would be the one indicated for future studies about the synthetic usefulness of the anionic cyclodehydrogenation mechanism to obtain new bay-perylene compounds.
Experimental Section
Computational Modeling
DFT calculations were carried out using Gaussian 09 package Rev.E.01.35 All calculations reported here were carried out with the hybrid-GGA functionals B3LYP20 and ω-B97XD21 at the 6-31+G(d) basis set with the polarizable continuum model (IEF-PCM)22 by employing toluene as a solvent (ϵ = 2.37). Calculations were performed with full geometry optimization. The characterization of stationary points was done by Hessian matrix calculations, with all positive eigenvalues for a minimum and only one negative eigenvalue for the TSs. XYZ coordinates of the optimized geometries are included in Section S4 of the SI.
Experimental Methodologies
Toluene (Carlo Erba) and solvents in general were used after distillation and stored under molecular sieves (4 Å) and an inert atmosphere. 1H-NMR, 13C-NMR, and all 2D NMR spectra were recorded using a 400 MHz Bruker nuclear magnetic resonance spectrometer. HRMS spectra were recorded using a Bruker, MicroTOF-Q II equipment, operated with an ESI source in the positive/negative mode, using nitrogen as a nebulizing and drying gas and 10 mM sodium formate as an internal standard. A gas chromatographic analysis was performed using a Varian GC with a flame ionization detector, which was equipped with a VF-5 MS, 30 m × 0.25 mm × 0.25 mm column. GC–MS analyses were carried out on a Shimadzu GC–MS QP5050 spectrometer, employing a 30 m, 0.12 mm DB-5 MS column.
Synthesis of Substrates
The compounds 1,1′-binaphthalene (1a),18 [1,1′-binaphthalen]-2-ol (4),28 [1,1′-binaphthalen]-2-amine,32 2-methoxy-1,1′-binaphthalene (1b),9a and N,N-dimethyl-[1,1′-binaphthalen]-2-amine (1d)9a were synthesized and purified following previously reported methods.
([1,1′-Binaphthalen]-2-yloxy)(tert-butyl)dimethylsilane (1c)
It was synthesized following a typical O-protection reaction by silanes.29a [1,1′-Binaphthalen]-2-ol (4) purified by column chromatography from previous synthesis (190 mg, 0.7 mmol ca., 1 equiv), 4-dimethylaminopyridine (8.5 mg, 0.07 mmol, 0.1 equiv), and imidazole (76 mg, 1.12 mmol, 1.6 equiv) were dissolved in dichloromethane (2 mL). The solution was cooled to 0 °C. tert-Butyldimethylsilyl chloride (115.5 mg, 0.77 mmol, 1.1 equiv) was added and the solution was allowed to warm to room temperature. After stirring overnight, the reaction mixture was treated with water (5 mL), HCl (35% in H2O, 0.2 mL), and ethyl acetate (5 mL). The organic layer was collected, and the aqueous layer was extracted with ethyl acetate twice (2 × 5 mL). The combined organic layers were washed with H2O, dried over MgSO4, and filtered. The filtrate was concentrated and purified by column chromatography (hexane/ethyl acetate, 99:1) to provide (1c) as a white solid (135 mg, 35% yield referred to the starting material for the synthesis of [1,1′-binaphthalen]-2-ol). M. p.: 139.0–141.1 °C. 1H NMR (400 MHz, CDCl3) δ 7.96–7.89 (m, 4H); 7.63–7.59 (m, 1H); 7.50–7.46 (m, 2H); 7.43–7.25 (m, 6H); 0.48 (s, 9H, 3 × CH3); −0.09 (s, 3H, Si–CH3); −0.09 (s, 3H, Si–CH3). 13C NMR (100 MHz, CDCl3) δ 151.0 (Cq); 135.1 (Cq); 134.7 (Cq); 133.9 (Cq); 133.2 (Cq); 129.4 (Cq); 129.2 (CAr–H); 129.0 (CAr–H); 128.1 (CAr–H); 127.9 (CAr–H); 127.7 (CAr–H); 126.7 (CAr–H); 126.3 (CAr–H); 125.9 (CAr–H); 125.8 (CAr–H); 125.7 (CAr–H); 125.7 (Cq); 125.7 (CAr–H); 125.5 (CAr–H); 123.7 (CAr–H); 121.1 (CAr–H); 25.2 (3 × CH3); 17.7 (C–(CH3)3); −4.3 (2 × Si–CH3). HRMS (ESI-TOF) m/z [M + Na]+ calcd for C26H28OSiNa: 407.1802; found: 407.1814. MS (EI): m/z 384 (15%), 327 (100%), 311 (21%), 252 (17%).
General Procedure for Anionic Cyclodehydrogenation Reactions
Into a previously dried 15 mL Schlenk tube flask equipped with a nitrogen inlet and a magnetic stirrer, 2 mL of a 0.11 M solution of substrate in dried toluene was added. The corresponding equivalents of potassium were added, and the temperature was adjusted according to the experimental setup. The first step of the workup consisted of the dropwise addition of an I2 (3 equiv) solution in dried toluene. A change in color and bubbles were observed. It was left to stir for 1 h. The second step was the very slow addition of a few mL of ethanol and the system was exposed to the air atmosphere. Finally, 1 mL of 10% of Na2S2O3 solution was added and the suspension was left to stir overnight. Extraction of organic compounds was done with CH2Cl2 (3 × 10 mL). The CH2Cl2 extract was washed twice. The organic extract thus obtained was dried over MgSO4. After filtration, the organic solvent was eliminated under a reduced pressure. Purification of the organic crude was carried out by column chromatography employing silica gel and hexane/ethyl acetate as eluents.
1,1′-Binaphthalene (1a)
It was purified by column chromatography with hexane/diethyl ether (9.9:0.1) as a white solid (90% yield).361H NMR (400 MHz, CDCl3) δ 7.97–7.94 (m, 4H); 7.61–7.58 (m, 2H); 7.51–7.46 (m, 4H); 7.40 (d, J = 8.4 Hz, 2H); 7.31–7.27 (m, 2H). MS (EI): m/z 254 (86%), 253 (100%), 252 (80%), 239 (18%), 126 (50%), 113 (25%).
Perylene (2a)
The product was purified as a pale-yellow solid by semipreparative TLC using hexane/ethyl acetate (90:10) as an eluent.9a1H NMR (400 MHz, CDCl3) δ 8.19 (d, J = 7.8 Hz, 4H); 7.68 (d, J = 8.0 Hz, 4H); 7.48 (t, J = 7.7 Hz, 4H). 13C NMR (100 MHz, CDCl3) δ 134.9 (2 × Cq); 131.4 (2 × Cq); 129.0 (4 × Cq); 128.0 (4 × CAr–H); 126.7 (4 × CAr–H); 120.4 (4 × CAr–H). MS (EI): m/z 252 (100%), 250 (19%), 125 (18%), 113 (7%).
N,N-Dimethylperylene-1-amine (2d)
The compound was purified as an orange oily solid by semipreparative TLC using as eluent a solvent gradient of hexane/ethyl acetate (100:0 → 90:10).9a Isolated yield: 50% (27 mg). 1H NMR (400 MHz, (CD3)2CO) δ 9.21 (dd, 1J = 7.8, 2J = 1.0 Hz, 1H); 8.21 (dd, 1J = 7.5, 2J = 1.1 Hz, 2H); 7.73–7.64 (m, 4H); 7.54–7.46 (m, 3H); 7.38 (t, 1J = 7.8 Hz, 1H); 2.85 (s, 6H). 13C NMR (100 MHz, (CD3)2CO) δ 151.0 (Cq, C–N); 135.6 (Cq); 132.5 (Cq); 132.4 (Cq); 131.9 (Cq); 131.6 (Cq); 131.6 (Cq); 130.8 (Cq); 129.5 (CAr–H); 128.5 (CAr–H); 128.2 (CAr–H); 127.5 (CAr–H); 127.1 (CAr–H); 127.0 (CAr–H); 125.2 (CAr–H); 124.5 (CAr–H); 122.1 (CAr–H); 121.5 (CAr–H); 120.1 (CAr–H); 119.6 (Cq); 43.4 (2C, CH3). MS (EI): m/z 295 (100%), 280 (34%), 265 (41%), 264 (23%), 146 (34%), 125 (34%). The 2D NMR spectra are shown in the SI.
[1,1′-Binaphthalen]-2-ol (4)
It was purified by column chromatography with hexane/diethyl ether (9:1) as a pale-yellow solid (58% isolated yield).281H NMR (400 MHz, CDCl3) δ 8.09 (s, 1H, OH); 8.01 (d, J = 8.2 Hz, 2H); 7.94 (d, J = 8.8 Hz, 1H); 7.89 (d, J = 8.2 Hz, 1H); 7.67–7.64 (m, 1H); 7.52–7.47 (m, 2H); 7.38 (d, J = 8.8 Hz, 1H); 7.32–7.27 (m, 3H); 7.23–7.19 (m, 1H); 7.01 (d, J = 8.4 Hz, 1H). MS (EI): m/z 270 (100%), 253 (32%), 239 (43%), 135 (7%), 126 (20%), 113 (11%).
Benzo[j]fluoranthene (5)
The product was purified as a yellow solid by semipreparative TLC using hexane/ethyl acetate (90:10) as an eluent.371H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 8.4 Hz, 1H); 8.46 (d, J = 7.0 Hz, 1H); 8.06 (d, J = 8.2 Hz, 1H); 8.01 (d, J = 6.8 Hz, 1H); 7.93 (d, J = 8.2 Hz, 1H); 7.88 (d, J = 8.2 Hz, 2H); 7.87 (d, J = 8.2 Hz, 1H); 7.73–7.60 (m, 3H); 7.49 (t, J = 8.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 138.0 (Cq); 137.9 (Cq); 137.3 (Cq); 136.7 (Cq); 134.4 (Cq); 134.2 (Cq); 130.7 (Cq); 129.8 (Cq); 129.5 (CAr–H); 128.5 (CAr–H); 128.3 (CAr–H); 128.0 (CAr–H); 127.5 (CAr–H); 127.1 (CAr–H); 127.1 (CAr–H); 125.4 (CAr–H); 124.4 (CAr–H); 124.3 (CAr–H); 121.0 (CAr–H); 120.0 (CAr–H). MS (EI): m/z 252 (100%), 250 (19%), 125 (18%), 113 (7%). The 2D NMR spectra are shown in the SI.
Acknowledgments
This work was partly financially supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba (SECyT), and the Agencia Nacional de Promoción Científica y Técnica (ANPCyT). J.L.B. gratefully acknowledges a fellowship from CONICET and SECyT. L.B.J. acknowledges Carlos R. Medrano for his help in experimental stuff. This work used computational resources from CCAD—Universidad Nacional de Córdoba (https://ccad.unc.edu.ar/), which are part of SNCAD—MinCyT, República Argentina.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02017.
Computational modeling information (determination of electronic state and optimized XYZ coordinates) and experimental description (synthesis and spectroscopic data) (PDF)
The authors declare no competing financial interest.
Dedication
In memory of Adriana B. Pierini (1953–2016).
Supplementary Material
References
- a Enrichi F.; Righini G. C.. Solar Cells and Light Management: Materials, Strategies and Sustainability; Elsevier: Amsterdam, 2020. [Google Scholar]; b Yersin H.Highly efficient OLEDs: Materials based on thermally activated delayed fluorescence; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019. [Google Scholar]; c Li Z. R.Organic light-emitting materials and devices; CRC Press: Boca Raton, 2015. [Google Scholar]
- Hong G.; Gan X.; Leonhardt C.; Zhang Z.; Seibert J.; Busch J. M.; Brase S. A Brief History of OLEDs-Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, e2005630 10.1002/adma.202005630. [DOI] [PubMed] [Google Scholar]
- a Kalyani N. T.; Dhoble S. J. Novel materials for fabrication and encapsulation of OLEDs. Renewable Sustainable Energy Rev. 2015, 44, 319–347. 10.1016/j.rser.2014.11.070. [DOI] [Google Scholar]; b Kalyani N. T.; Dhoble S. J. Organic Light Emitting Diodes: energy saving lighting technology-A review. Renewable Sustainable Energy Rev. 2012, 16, 2696–2723. 10.1016/j.rser.2012.02.021. [DOI] [Google Scholar]
- a Wadsworth A.; Moser M.; Marks A.; Little M. S.; Gasparini N.; Brabec C. J.; Baran D.; McCulloch I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019, 48, 1596–1625. 10.1039/c7cs00892a. [DOI] [PubMed] [Google Scholar]; b Yan C.; Barlow S.; Wang Z.; Yan H.; Jen A. K. Y.; Marder S. R.; Zhan X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. 10.1038/natrevmats.2018.3. [DOI] [Google Scholar]
- a Li G.; Yang W.; Wang S.; Liu T.; Yan C.; Li G.; Zhang Y.; Li D.; Wang X.; Hao P.; Li J.; Huo L.; Yan H.; Tang B. Methane-perylene diimide-based small molecule acceptors for high efficiency non-fullerene organic solar cells. J. Mater. Chem. C 2019, 7, 10901–10907. 10.1039/C9TC03457A. [DOI] [Google Scholar]; b Markiewicz J. T.; Wudl F. Perylene, Oligorylenes, and Aza-Analogs. ACS Appl. Mater. Interfaces 2015, 7, 28063–28085. 10.1021/acsami.5b02243. [DOI] [PubMed] [Google Scholar]
- a Bhargava Rao B.; Wei J. R.; Lin C. H. New synthetic routes to Z-shape functionalized perylenes. Org. Lett. 2012, 14, 3640–3643. 10.1021/ol3014667. [DOI] [PubMed] [Google Scholar]; b Ilhan F.; Tyson D. S.; Stasko D. J.; Kirschbaum K.; Meador M. A. Twisted, Z-shaped perylene bisimide. J. Am. Chem. Soc. 2006, 128, 702–703. 10.1021/ja056912o. [DOI] [PubMed] [Google Scholar]
- a Sample C. S.; Goto E.; Handa N. V.; Page Z. A.; Luo Y.; Hawker C. J. Modular synthesis of asymmetric rylene derivatives. J. Mater. Chem. C 2017, 5, 1052–1056. 10.1039/C6TC05139A. [DOI] [Google Scholar]; b Sakamoto T.; Pac C. A ″green″ route to perylene dyes: direct coupling reactions of 1,8-naphthalimide and related compounds under mild conditions using a ″new″ base complex reagent, t-BuOK/DBN. J. Org. Chem. 2001, 66, 94–98. 10.1021/jo0010835. [DOI] [PubMed] [Google Scholar]
- a Pigulski B.; Ximenis M.; Shoyama K.; Würthner F. Synthesis of polycyclic aromatic hydrocarbons by palladium-catalysed [3 + 3] annulation. Org. Chem. Front. 2020, 7, 2925–2930. 10.1039/D0QO00968G. [DOI] [Google Scholar]; b Shoyama K.; Mahl M.; Seifert S.; Wurthner F. A General Synthetic Route to Polycyclic Aromatic Dicarboximides by Palladium-Catalyzed Annulation Reaction. J. Org. Chem. 2018, 83, 5339–5346. 10.1021/acs.joc.8b00301. [DOI] [PubMed] [Google Scholar]; c Dyker G. Transition metal catalyzed annulation reactions. Part 3. Palladium-catalyzed annulation reactions of 1,8-diiodonaphthalene. J. Org. Chem. 2002, 58, 234–238. 10.1021/jo00053a042. [DOI] [Google Scholar]; d Vyskočil Š.; Meca L.; Tišlerová I.; Císařová I.; Polášek M.; Harutyunyan S. R.; Belokon Y. N.; Stead R. M. J.; Farrugia L.; Lockhart S. C.; Mitchell W. L.; Kočovský P. 2,8′-Disubstituted-1,1′-Binaphthyls: A New Pattern in Chiral Ligands. Chem. – Eur. J. 2002, 8, 4633–4648. . [DOI] [PubMed] [Google Scholar]
- a Camargo Solórzano P.; Baumgartner M. T.; Puiatti M.; Jimenez L. B. Arenium cation or radical cation? An insight into the cyclodehydrogenation reaction of 2-substituted binaphthyls mediated by Lewis acids. RSC Adv. 2020, 10, 21974–21985. 10.1039/d0ra04213g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rickhaus M.; Belanger A. P.; Wegner H. A.; Scott L. T. An oxidation induced by potassium metal. Studies on the anionic cyclodehydrogenation of 1,1′-binaphthyl to perylene. J. Org. Chem. 2010, 75, 7358–7364. 10.1021/jo101635z. [DOI] [PubMed] [Google Scholar]; c Gryko D. T.; Piechowska J.; Galezowski M. Strongly emitting fluorophores based on 1-azaperylene scaffold. J. Org. Chem. 2010, 75, 1297–1300. 10.1021/jo902443s. [DOI] [PubMed] [Google Scholar]; d Schlichting P.; Rohr U.; Müllen K. Easy synthesis of liquid crystalline perylene derivatives. J. Mater. Chem. 1998, 8, 2651–2655. 10.1039/a804332i. [DOI] [Google Scholar]; e Jiang Z.; Zhou S.; Jin W.; Zhao C.; Liu Z.; Yu X. Synthesis, Structure, and Photophysical Properties of BN-Embedded Analogue of Coronene. Org. Lett. 2022, 24, 1017–1021. 10.1021/acs.orglett.1c04161. [DOI] [PubMed] [Google Scholar]
- a van Dijk J. T. M.; Hartwijk A.; Bleeker A. C.; Lugtenburg J.; Cornelisse J. Gram Scale Synthesis of Benzo[ghi]perylene and Coronene. J. Org. Chem. 1996, 61, 1136–1139. 10.1021/jo951555t. [DOI] [Google Scholar]; b Langhals H.; Grundner S. Fluoreszenzfarbstoffe mit Fünfring-Carbonsäureimid-Strukturen. Chem. Ber. 1986, 119, 2373–2376. 10.1002/cber.19861190728. [DOI] [Google Scholar]
- Nowak-Król A.; Würthner F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. 10.1039/C8QO01368C. [DOI] [Google Scholar]
- a Kurpanik A.; Matussek M.; Lodowski P.; Szafraniec-Gorol G.; Krompiec M.; Krompiec S. Diels-Alder Cycloaddition to the Bay Region of Perylene and Its Derivatives as an Attractive Strategy for PAH Core Expansion: Theoretical and Practical Aspects. Molecules 2020, 25, 5373–5424. 10.3390/molecules25225373. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nakamuro T.; Kumazawa K.; Ito H.; Itami K. Bay-Region-Selective Annulative-Extension (APEX) of Perylene Diimides with Arynes. Synlett 2019, 30, 423–428. [Google Scholar]; c Swager T.; Yoshinaga K. Fluorofluorescent Perylene Bisimides. Synlett 2018, 29, 2509–2514. 10.1055/s-0037-1610224. [DOI] [Google Scholar]; d Pagoaga B.; Mongin O.; Caselli M.; Vanossi D.; Momicchioli F.; Blanchard-Desce M.; Lemercier G.; Hoffmann N.; Ponterini G. Optical and photophysical properties of anisole- and cyanobenzene-substituted perylene diimides. Phys. Chem. Chem. Phys. 2016, 18, 4924–4941. 10.1039/c5cp07758c. [DOI] [PubMed] [Google Scholar]
- a Merz J.; Steffen A.; Nitsch J.; Fink J.; Schurger C. B.; Friedrich A.; Krummenacher I.; Braunschweig H.; Moos M.; Mims D.; Lambert C.; Marder T. B. Synthesis, photophysical and electronic properties of tetra-donor- or acceptor-substituted ortho-perylenes displaying four reversible oxidations or reductions. Chem. Sci. 2019, 10, 7516–7535. 10.1039/C9SC02420D. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Li B.; Peng W.; Luo S.; Jiang C.; Guo J.; Xie S.; Hu Y.; Zhang Y.; Zeng Z. Diagonally pi-Extended Perylene-Based Bis(heteroacene) for Chiroptical Activity and Integrating Luminescence with Carrier-Transporting Capability. Org. Lett. 2019, 21, 1417–1421. 10.1021/acs.orglett.9b00152. [DOI] [PubMed] [Google Scholar]; c Wu J.; He D.; Wang Y.; Su F.; Guo Z.; Lin J.; Zhang H. J. Selective Ortho-pi-Extension of Perylene Diimides for Rylene Dyes. Org. Lett. 2018, 20, 6117–6120. 10.1021/acs.orglett.8b02557. [DOI] [PubMed] [Google Scholar]; d Li Y.; Hong Y.; Guo J.; Huang X.; Wei H.; Zhou J.; Qiu T.; Wu J.; Zeng Z. Bay- and Ortho-Octasubstituted Perylenes. Org. Lett. 2017, 19, 5094–5097. 10.1021/acs.orglett.7b02370. [DOI] [PubMed] [Google Scholar]
- a Hu M.; Sukhanov A. A.; Zhang X.; Elmali A.; Zhao J.; Ji S.; Karatay A.; Voronkova V. K. Spiro Rhodamine-Perylene Compact Electron Donor-Acceptor Dyads: Conformation Restriction, Charge Separation, and Spin-Orbit Charge Transfer Intersystem Crossing. J. Phys. Chem. B 2021, 125, 4187–4203. 10.1021/acs.jpcb.1c02071. [DOI] [PubMed] [Google Scholar]; b Ahn M.; Kim M. J.; Cho D. W.; Wee K. R. Electron Push-Pull Effects on Intramolecular Charge Transfer in Perylene-Based Donor-Acceptor Compounds. J. Org. Chem. 2021, 86, 403–413. 10.1021/acs.joc.0c02149. [DOI] [PubMed] [Google Scholar]; c Ahn M.; Kim M. J.; Wee K. R. Electron Push-Pull Effects in 3,9-Bis(p-(R)-diphenylamino)perylene and Constraint on Emission Color Tuning. J. Org. Chem. 2019, 84, 12050–12057. 10.1021/acs.joc.9b01849. [DOI] [PubMed] [Google Scholar]; d Sharma V.; Chandra F.; Sahoo D.; Koner A. L. Efficient Microwave-Assisted Synthesis of Sonogashira-Coupled Perylene Monoimide Derivatives: Impact of Electron-Donating Groups on Optoelectronic Properties. Eur. J. Org. Chem. 2017, 2017, 6901–6905. 10.1002/ejoc.201701310. [DOI] [Google Scholar]; e Chou H. H.; Liu Y. C.; Fang G.; Cao Q. K.; Wei T. C.; Yeh C. Y. Structurally Simple and Easily Accessible Perylenes for Dye-Sensitized Solar Cells Applicable to Both 1 Sun and Dim-Light Environments. ACS Appl. Mater. Interfaces 2017, 9, 37786–37796. 10.1021/acsami.7b11784. [DOI] [PubMed] [Google Scholar]; f Qi Q.; Wang X.; Fan L.; Zheng B.; Zeng W.; Luo J.; Huang K. W.; Wang Q.; Wu J. N-annulated perylene-based push-pull-type sensitizers. Org. Lett. 2015, 17, 724–727. 10.1021/ol503749f. [DOI] [PubMed] [Google Scholar]
- Solodovnikov S. P.; Ioffe S. T.; Zaks Y. B.; Kabachnik M. I. On the formation of perylene when metallic potassium reacts with 1,1′-dinaphthyl in 1,2-dimethoxyethane. Russ. Chem. Bull. 1968, 17, 442–443. 10.1007/BF00908472. [DOI] [Google Scholar]
- Uchida Y.; Hirose T.; Nakashima T.; Kawai T.; Matsuda K. Synthesis and Photophysical Properties of a 13,13’-Bibenzo[b]perylenyl Derivative as a π-Extended 1,1’-Binaphthyl Analog. Org. Lett. 2016, 18, 2118–2121. 10.1021/acs.orglett.6b00747. [DOI] [PubMed] [Google Scholar]
- a Biagiotti G.; Perini I.; Richichi B.; Cicchi S. Novel Synthetic Approach to Heteroatom Doped Polycyclic Aromatic Hydrocarbons: Optimizing the Bottom-Up Approach to Atomically Precise Doped Nanographenes. Molecules 2021, 26, 6306–6346. 10.3390/molecules26206306. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kawahara K. P.; Matsuoka W.; Ito H.; Itami K. Synthesis of Nitrogen-Containing Polyaromatics by Aza-Annulative π-Extension of Unfunctionalized Aromatics. Angew. Chem., Int. Ed. 2020, 59, 6383–6388. 10.1002/anie.201913394. [DOI] [PubMed] [Google Scholar]; c Firmansyah D.; Banasiewicz M.; Deperasińska I.; Makarewicz A.; Kozankiewicz B.; Gryko D. Vertically π-Expanded Imidazo[1,2-a]pyridine: The Missing Link of the Puzzle. Chem. – Asian J. 2014, 9, 2483–2493. 10.1002/asia.201402201. [DOI] [PubMed] [Google Scholar]
- Guo W.; Faggi E.; Sebastián R. M.; Vallribera A.; Pleixats R.; Shafir A. Direct Arylation of Oligonaphthalenes Using PIFA/BF3·Et2O: From Double Arylation to Larger Oligoarene Products. J. Org. Chem. 2013, 78, 8169–8175. 10.1021/jo401001k. [DOI] [PubMed] [Google Scholar]
- Different combinations of solvents/reducing agents were proposed for this reductive reaction however all of them showed poor reaction yields in general (10% or less). Ref (9b).
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Chai J. D.; Head-Gordon M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]
- Tomasi J.; Mennucci B.; Cammi R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
- For the calculations, G09 Rev-E.01 was used. In the case of OS, the keyword guess(mix,always) was added in the modeling. For further details, check the discussion in the Supporting Information, page SI-3.
- The obtained reaction barriers are similar to the recently computed barriers for the cyclodehydrogenation reaction of 2-substituted binaphthyls, mediated by Lewis acids, for the synthesis of substituted perylenes. Ref (9a).
- Ayalon A.; Rabinovitz M. Reductive ring closure of helicenes. Tetrahedron Lett. 1992, 33, 2395–2398. 10.1016/S0040-4039(00)74221-3. [DOI] [Google Scholar]
- a Hazimeh H.; Mattalia J.-M.; Marchi-Delapierre C.; Barone R.; Nudelman N. S.; Chanon M. Radical clocks and electron transfer. Comparison of crown ether effects on the reactivity of potassium and magnesium towards 1-bromo-2-(3-butenyl)benzene. The incidence of homogeneous versus heterogeneous electron transfer on selectivity. J. Phys. Org. Chem. 2005, 18, 1145–1160. 10.1002/poc.986. [DOI] [Google Scholar]; b Thornton T. A.; Woolsey N. F.; Bartak D. E. Carbon-Oxygen Bond-Cleavage Reactions by Electron Transfer. 3. Electrochemical Formation and Decomposition of the Diphenyl Ether Radical Anion. J. Am. Chem. Soc. 1986, 108, 6497–6502. 10.1021/ja00281a008. [DOI] [Google Scholar]
- Azzena U.; Denurra T.; Melloni G. Electron-Transfer-Induced Reductive Demethoxylation of Anisole: Evidence for Cleavage of a Radical Anion. J. Org. Chem. 1992, 57, 1444–1448. 10.1021/jo00031a022. [DOI] [Google Scholar]
- Pierini A. B.; Baumgartner M. T.; Rossi R. A. Regiochemistry of the coupling of aryl radicals with nucleophiles derived from the naphthyl system. Experimental and theoretical studies. J. Org. Chem. 1991, 56, 580–586. 10.1021/jo00002a019. [DOI] [Google Scholar]
- a Baker M. S.; Phillips S. T. A Two-Component Small Molecule System for Activity-Based Detection and Signal Amplification: Application to the Visual Detection of Threshold Levels of Pd(II). J. Am. Chem. Soc. 2011, 133, 5170–5173. 10.1021/ja108347d. [DOI] [PubMed] [Google Scholar]; b Furniss B. S.; Hannaford A. J.; Smith P. W. G.; Tatchell A. R.. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Scientific & Technical. John Wiley & Sons Inc.(New York): Harlow., 1989. [Google Scholar]
- The extremely basic reaction conditions deprotonated the hydroxyl group, preventing the reduction of the anionic substrates and further cyclization reactions.
- a Azzena U.; Dessanti F.; Melloni G.; Pisano L. Electron-transfer-induced reductive dealkoxylation of alkyl aryl ethers. III. Reductive cleavage of methoxy-substituted N,N- dimethylanilines (N,N-dimethylanisidines). ARKIVOC 2002, V, 181–188. 10.3998/ark.5550190.0003.520. [DOI] [Google Scholar]; b Azzena U.; Dessanti F.; Melloni G.; Pisano L. Single electron transfer reductive cleavage of the aryl-nitrogen bond in phenyl-substituted dimethylanilines. Tetrahedron Lett. 1999, 40, 8291–8293. 10.1016/S0040-4039(99)01758-X. [DOI] [Google Scholar]
- Pierini A. B.; Baumgartner M. T.; Rossi R. A. Photostimulated reactions of haloarenes with 2-naphtylamide ions. A facile synthesis of 1-aryl-2-naphthylamines. Tetrahedron Lett. 1987, 28, 4653–4656. 10.1016/S0040-4039(00)96588-2. [DOI] [Google Scholar]
- Amick A. W.; Martin S. E. Use of External Radical Sources in Flash Vacuum Pyrolysis to Facilitate Cyclodehydrogenation Reactions in Polycyclic Aromatic Hydrocarbons. Aust. J. Chem. 2014, 67, 1338–1343. 10.1071/CH14289. [DOI] [Google Scholar]
- a Genesty M.; Degrand C. Preparation of S42– polysulfide from a sacrificial sulfur cathode and its use as a nucleophile in an electrochemically induced SRN1 substitution reaction. New J. Chem. 1998, 349–354. 10.1039/a800523k. [DOI] [Google Scholar]; b Combellas C.; Dellerue S.; Mathey G.; Thiébault A. Nucleophilic properties of thiourea towards aromatic halides. Tetrahedron Lett. 1997, 38, 539–542. 10.1016/S0040-4039(96)02366-0. [DOI] [Google Scholar]; c Rossi R. A.; Pierini A. B.; Peñeñory A. B. Nucleophilic Substitution Reactions by Electron Transfer. Chem. Rev. 2003, 103, 71–167. 10.1021/cr960134o. [DOI] [PubMed] [Google Scholar]
- Gaussian 09, Revision E.01; M. J., Frisch, et al. ; Gaussian, Inc.: Wallingford CT, 2013. Full citation included in the SI. [Google Scholar]
- Liu Y.; Bergès J.; Zaid Y.; Chahdi F. O.; Lee A. V. D.; Harakat D.; Clot E.; Jaroschik F.; Taillefer M. Aerobic and Ligand-Free Manganese-Catalyzed Homocoupling of Arenes or Aryl Halides via in Situ Formation of Aryllithiums. J. Org. Chem. 2019, 84, 4413–4420. 10.1021/acs.joc.8b02834. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M. H. M.; Tazawa K.; Manabe K. Three-Step Synthesis of Fluoranthenes through Pd-Catalyzed Inter- and Intramolecular C–H Arylation. J. Org. Chem. 2016, 81, 3967–3974. 10.1021/acs.joc.6b00553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.