Abstract
Hypothesis:
Bilateral cochlear-implant (BI-CI) users will have a range of interaural insertion-depth mismatch because of different array placement or characteristics. Mismatch will be larger for electrodes located near the apex or outside scala tympani, or for arrays that are a mix of pre-curved and straight types.
Background:
Brainstem superior olivary-complex neurons are exquisitely sensitive to interaural-difference cues for sound localization. Because these neurons rely on interaurally place-of-stimulation-matched inputs, interaural insertion-depth or scalar-location differences for BI-CI users could cause interaural place-of-stimulation mismatch that impairs binaural abilities.
Methods:
Insertion depths and scalar locations were calculated from temporal-bone computed-tomography scans for 107 BI-CI users (27 Advanced Bionics, 62 Cochlear, 18 MED-EL).
Results:
Median interaural insertion-depth mismatch was 23.4° or 1.3 mm. Mismatch in the estimated clinically relevant range expected to impair binaural processing (>75° or 3 mm) occurred for 13–19% of electrode pairs overall, and for at least three electrode pairs for 23–37% of subjects. There was a significant three-way interaction between insertion depth, scalar location, and array type. Interaural insertion-depth mismatch was largest for apical electrodes, for electrode pairs in two different scala, and for arrays that were both pre-curved.
Conclusion:
Average BI-CI interaural insertion-depth mismatch was small; however, large interaural insertion-depth mismatch—with the potential to degrade spatial hearing—occurred frequently enough to warrant attention. For new BI-CI users, improved surgical techniques to avoid interaural insertion-depth and scalar mismatch are recommended. For existing BI-CI users with interaural insertion-depth mismatch, interaural alignment of clinical frequency tables might reduce negative spatial-hearing consequences.
I. INTRODUCTION
The purpose of a single (unilateral) cochlear implant (CI) is to partially restore sound and speech perception to people with poor hearing, typically those with bilateral moderate to profound hearing loss. Bilateral CIs (BI-CIs) are becoming increasingly prevalent1,2. The primary purpose of BI-CIs is to convey cues for auditory spatial perception. Although BI-CI users have better sound localization and speech recognition in the presence of competing sounds compared to unilateral CI users3–6, the spatial-hearing benefits that they receive from two ears are far less than those experienced by normal-hearing (NH) listeners7–10, and they continue to struggle to communicate in noisy environments11–13.
In typical auditory systems, binaural sensitivity is computed in brainstem neurons that receive tonotopically symmetric input from the auditory periphery14–16. For BI-CIs to convey maximally useful binaural cues, it is imperative to minimize interaural place-of-stimulation mismatch14,17–21. This can be partially achieved by surgically matching the insertion depths (i.e., minimizing interaural insertion-depth mismatch) for the two CIs in a BI-CI user. In reality, perfectly matching insertion depths is difficult to achieve in complex CI surgeries due to a high prevalence of relatively shallow insertion depths22,23 and scalar translocations24. If information about the interaural insertion-depth mismatch were available, the audiologist could potentially realign the ears using asymmetric frequency mappings in the two CIs. In addition, interaural place-of-stimulation mismatch may be caused by mismatch in the scalar location of electrodes (i.e., interaural scalar mismatch). The electrode array is commonly inserted in Scala Tympani (ST) through the round window and is intended to remain entirely in ST25, without damaging the basilar membrane or Reissner’s membrane. The apical end of the electrode array can sometimes unintentionally puncture one or both membranes, producing electrodes at least partially located in Scala Media (SM) or Scala Vestibuli (SV)25–28. Finally, it is sometimes not possible to insert the array completely within the ST cavity, in which case the array can occupy the SM or SV cavities immediately upon initial insertion. While interaural insertion-depth mismatch for BI-CI users has most commonly been examined in small-N studies using psychophysical17–21,29,30 and electrophysiological techniques19, our understanding of the prevalence and characteristics of this mismatch is limited at the population level31, and interaural scalar mismatch has not been examined at all.
This study used computed-tomography (CT) scans and computer-model rendering32–34 to assess the extent to which interaural insertion-depth mismatch exists in a population of 107 BI-CI users. By characterizing the prevalence of interaural insertion-depth and scalar mismatch, and their dependence on array type and tonotopic location, the intent was to provide guidance to surgeons and audiologists in choosing array types and programming strategies to optimize BI-CI hearing outcomes. First, we hypothesized that BI-CI users would have a range of interaural insertion-depth mismatch because of different physical placements of the arrays or different array types in the two ears. Based on previous reports of tuning for binaural sensitivity17,30, we defined the estimated clinically relevant range for which binaural sensitivity is expected to be negatively affected to include any interaural insertion-depth mismatch >3 mm along an average basilar membrane (BM), which is equivalent to an angular difference of approximately 75°. The population standard deviation in insertion angle for a unilateral CI is (coincidentally) about 75°, based on the summary of the literature provided in Table I of Landsberger et al.22. If an individual BI-CI user’s two insertions were independently selected at random from this assumed normal distribution, the mismatch between the two arrays would have mean zero and be normally distributed with a standard deviation a factor of √2 larger, or 106°, and thus 48% of cases would have clinically relevant absolute mismatch (>75°). However, the cochleae for a given individual are interaurally symmetric, and thus the two insertion depths are not independent. We therefore hypothesized that the prevalence of clinically relevant interaural insertion-depth mismatch would be smaller than 48%.
Second, we hypothesized that scalar translocation might be prevalent in BI-CI users, particularly at the array’s apical end27,35. This should increase the prevalence of interaural scalar mismatch, predominantly in cases when the arrays follow different intended trajectories through the cochlea (i.e., they are a mix of pre-curved and straight types), which should in turn increase the amount of interaural insertion-depth mismatch. In other words, when considering the shape of the cochlea, the different array types, and different potential insertion trajectories, the geometry of the situation leads one to consider greater potential for interaural insertion-depth mismatch near the apex.
II. METHODS
Post-operative CT scan and image analysis32–34,36–38 was performed on 107 BI-CI users from Vanderbilt University and University of Maryland-College Park. The study population included 27 Advanced Bionics users (13 both pre-curved, 10 both straight, 4 mixed), 62 Cochlear Ltd. users (49 both pre-curved, 3 both straight, 10 mixed), and 18 MED-EL users (all both straight). Behavioral data for 19 of these BI-CI users (all Cochlear Ltd.) are reported in Bernstein et al.39
To determine the intra-cochlear locations of the electrodes, a patient-specific cochlea shapes were derived from the patient CT scans using a model that includes segmentation of the intra-cochlear anatomy32–34,36–38. This analysis quantifies the estimated electrode position in three dimensions. For the present analysis, we were interested in (1) the insertion angle based on a coordinate system defined by a line drawn between the round window and the modiolus and (2) the scalar location of the electrode (ST, SM, or SV)40.
Figure 1 shows CT scans from two example subjects, one with a small amount of interaural insertion-depth mismatch and no interaural scalar mismatch (top row) and one with a large amount of interaural insertion-depth mismatch and an interaural scalar mismatch (bottom row). Electrode scalar location was categorized as ST, SM, or SV based on the anatomical landmarks in the cochlea; ST and SV are shown as different colors in the leftmost and rightmost panels in Fig. 1.
Figure 1:
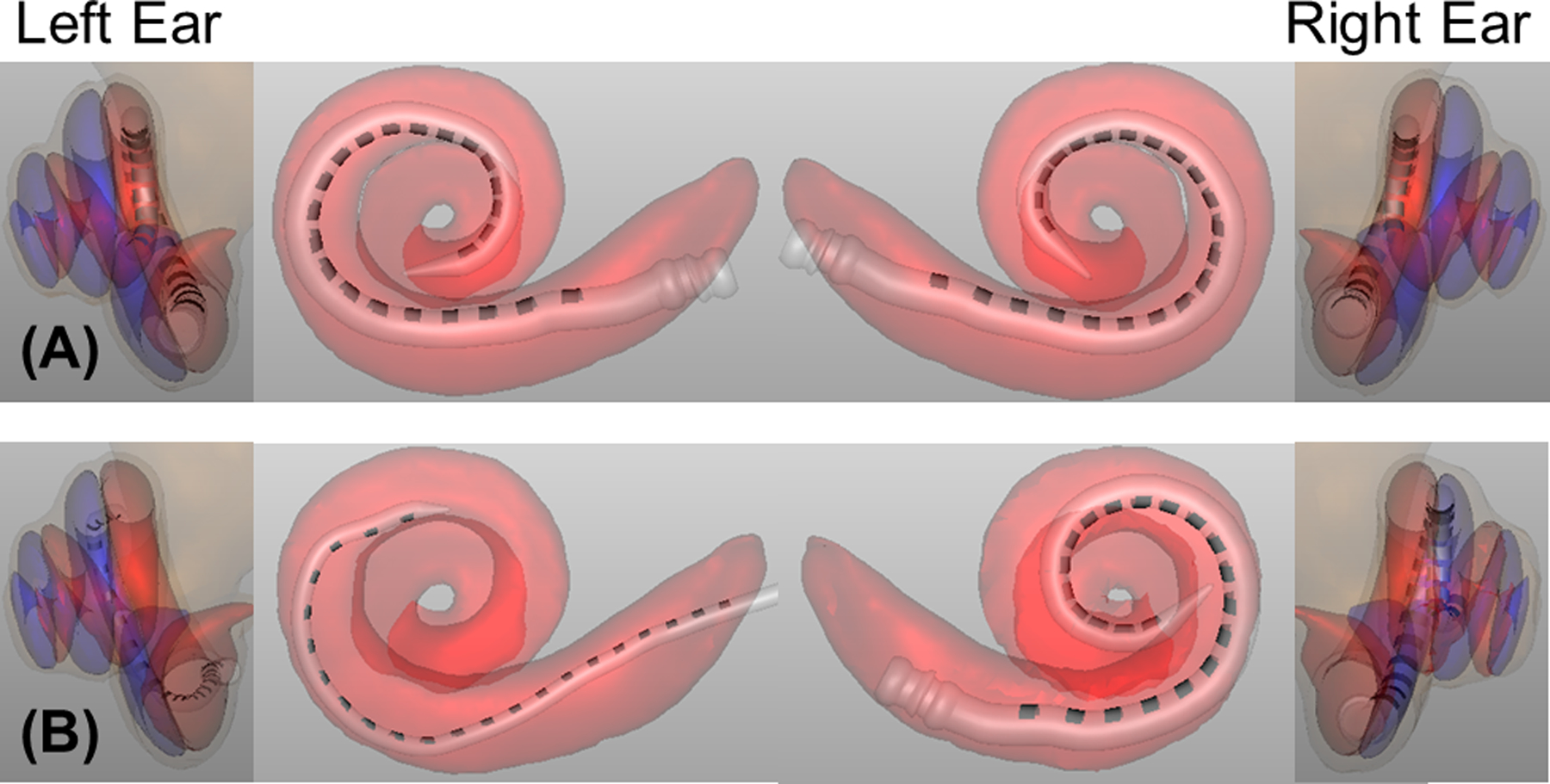
CT-scan model analyses for two example BI-CI users, with the left two columns showing the left ear and the right two columns showing the right ear. Insertion depth can be seen in the center panels. Scala location can be seen in the leftmost and rightmost panels; ST is shown in red and SV is shown in blue. The example BI-CI user in the top row (A) has a relatively small amount of interaural insertion-depth mismatch (center panels). The electrode arrays are both located in ST (red regions, leftmost and rightmost panels), and thus there is no interaural scalar mismatch. The example BI-CI user in the bottom row (B) has a relatively large amount of insertion-depth mismatch (the right-ear array is much deeper than the left-ear array) and there are numerous electrodes located in the blue regions outside of ST (i.e., some electrode pairs have an interaural scalar mismatch).
Interaural insertion-depth mismatch for number-matched electrode pairs was characterized in three ways. First, insertion depth was estimated in angular degrees around the cochlear spiral, with the modiolus at the center of the coordinate system and the round window defined as zero degrees. The statistical analyses of the dataset were based on this standard approach from the CI CT literature. Second, for comparison with previous estimates of the dependence of binaural sensitivity on mismatch17–21,29,30,41–44, the insertion depth was translated into BM distances (in millimeters) based on the spiral-ganglion correction45 to the Greenwood46 frequency-to-place map, assuming a 35-mm cochlear length. For both angular and BM distance, interaural insertion-depth mismatch was calculated as the absolute value of the difference for a given number-matched electrode pair. Third, to provide an acoustic-equivalent measure, interaural mismatch was calculated in octaves based on standard center-frequency (CF) allocation tables for each manufacturer. This interaural mismatch was calculated as the ratio of the CF for a given electrode to the effective CF for an electrode at the same insertion depth in the other ear, obtained by linearly interpolating the electrode insertion angles and allocation tables (log-frequency).
The dataset was statistically analyzed with a linear mixed-effects model (R version 4.0.3) using the buildmer (v1.7.1)47 and lme4 (v1.1–26)48 packages. The best-fitting model included interaural insertion-depth mismatch as the dependent variable and three fixed effects: insertion depth (in degrees, continuous variable), scalar location (categorical variable: ST-ST, SV-SV, and ST-SV), and array type (categorical variable: both pre-curved, both straight, and mixed). Electrodes located in SM were omitted because there were relatively few (7.3%) and because there are several different types of trauma that could be occurring for electrodes near SM, but without direct visualization of scalar structures the specific trauma is unclear. Electrodes located outside of the cochlea were also omitted. Random slopes and intercepts were included for scalar location by subject in the model of best fit chosen by the buildmer function. For the categorical variables, the model was referenced to the ST-ST and both pre-curved categories.
III. RESULTS
Figure 2 shows probability distribution functions (pdf; bars) and cumulative distribution functions (cdf; dotted curves) for interaural insertion-depth mismatch pooled across all electrodes and subjects. The median interaural insertion-depth mismatch was 23.4° (Fig. 2A), 1.3 mm (Fig. 2B), or 0.27 octaves (Fig. 2C). Figure 2A includes a vertical line at 75°, which denotes the lower boundary of the estimated clinically relevant range. Figure 2B includes a similar vertical line at 3 mm of interaural insertion-depth mismatch17–21,29,30,41–44. Interaural insertion-depth mismatch >75° occurred for 13.0% of electrode pairs and >3 mm occurred for 19.0% of electrode pairs. Mismatch >75° was observed for at least one electrode pair for 32.7% of the subjects, and for at least three electrode pairs for 23.4% of the subjects. Mismatch >3 mm was observed for at least one electrode pair for 43.0%, and for at least three electrode pairs for 37.4% of the subjects.
Figure 2:
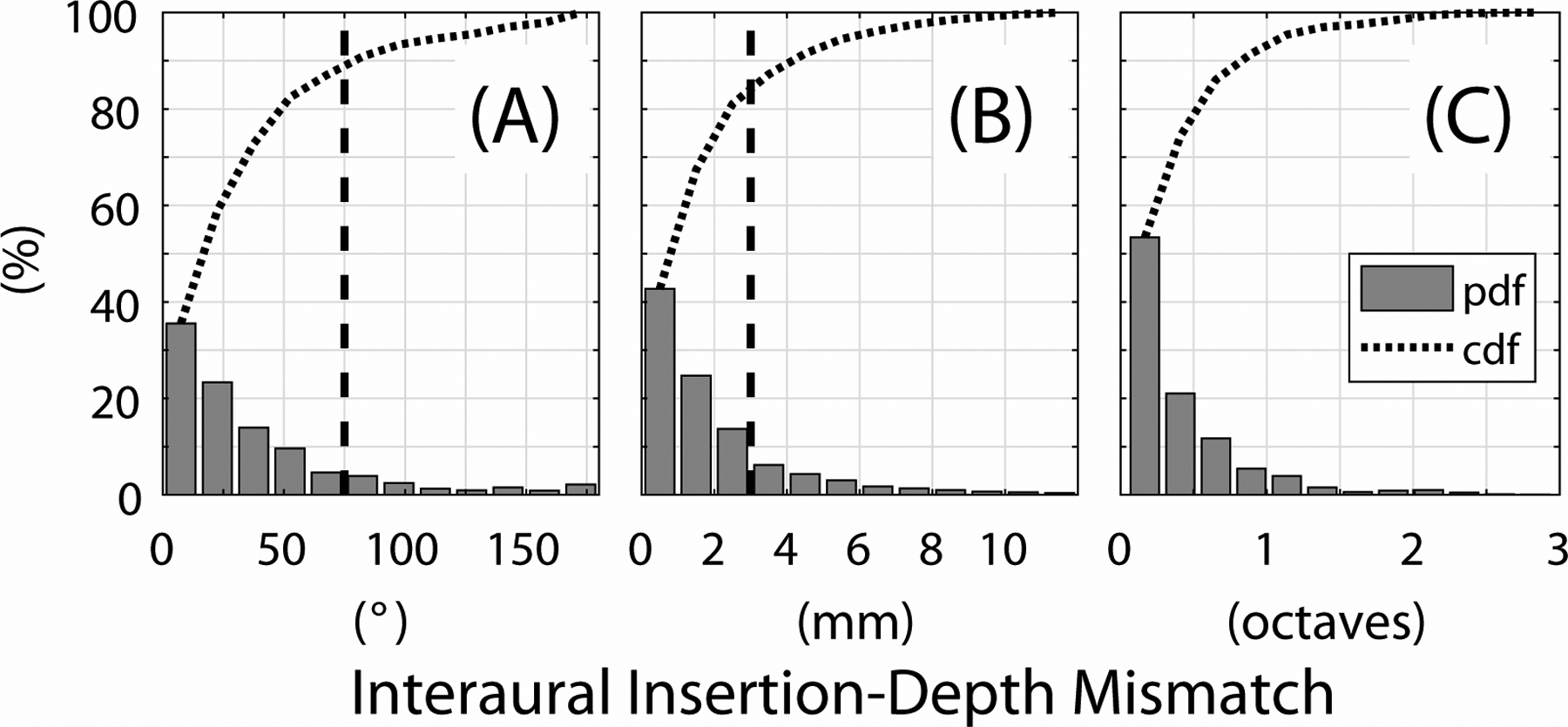
Distributions of interaural insertion-depth mismatch evaluated with respect to angle (degrees; panel A), distance (millimeters; panel B), and center frequency (octaves; panel C). Bars represent the probability distribution function (pdf) and the dotted curve shows the cumulative distribution function (cdf; i.e., the cumulative summation of the pdf). The vertical dashed lines depict an interaural insertion-depth mismatch of 75° (panel A) and 3 mm (panel B), respectively. These represent the lower boundary of the clinically relevant range where interaural insertion-depth mismatch is expected to degrade binaural perception.
Figure 3 shows the percentage of individual electrodes located in ST or SV as a function of the insertion depth. The percentage of scalar translocation increased with insertion depth. For depths <180°, fewer than 20% of electrodes were in SV. This increased to 25–35% for depths between 240°–420° and about 50% for depths >420°.
Figure 3:
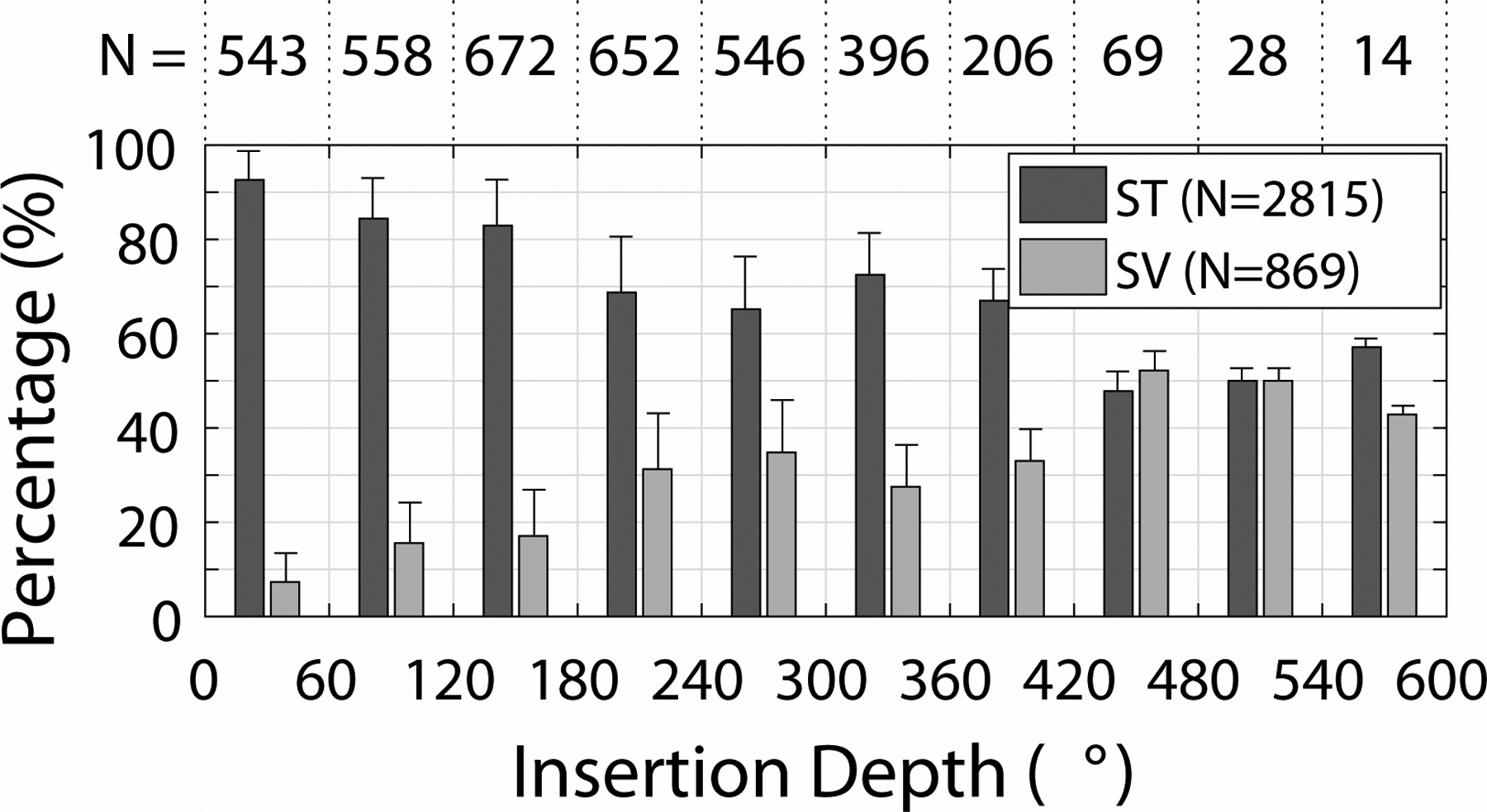
Histogram of individual electrodes located in Scala Tympani (ST) and Scala Vestibuli (SV) as a function of insertion depth in degrees, illustrated in terms of the percentage of electrodes in each scala for a given insertion depth range. The numbers (N) at the top of the plot show the number of electrodes at each depth, and the numbers inside the legend indicate the total number of electrodes in each category. Error bars show +1 standard deviation.
Figure 4 shows the absolute interaural insertion-depth mismatch as a function of the average insertion depth for each electrode pair, plotted separately for each combination of scalar location (rows) and array type (columns). Data are included for the 1715 pairs from 105 subjects. Figures 4E and 4F had insufficient counts to assess the relationship between interaural insertion-depth mismatch and insertion depth, with electrodes from only 1 or 2 subjects in each case. For the other categories, the fitted regression lines show a tendency for more interaural insertion-depth mismatch with greater insertion depth (i.e., positive slopes) when at least one of the arrays was pre-curved (left and right columns).
Figure 4:
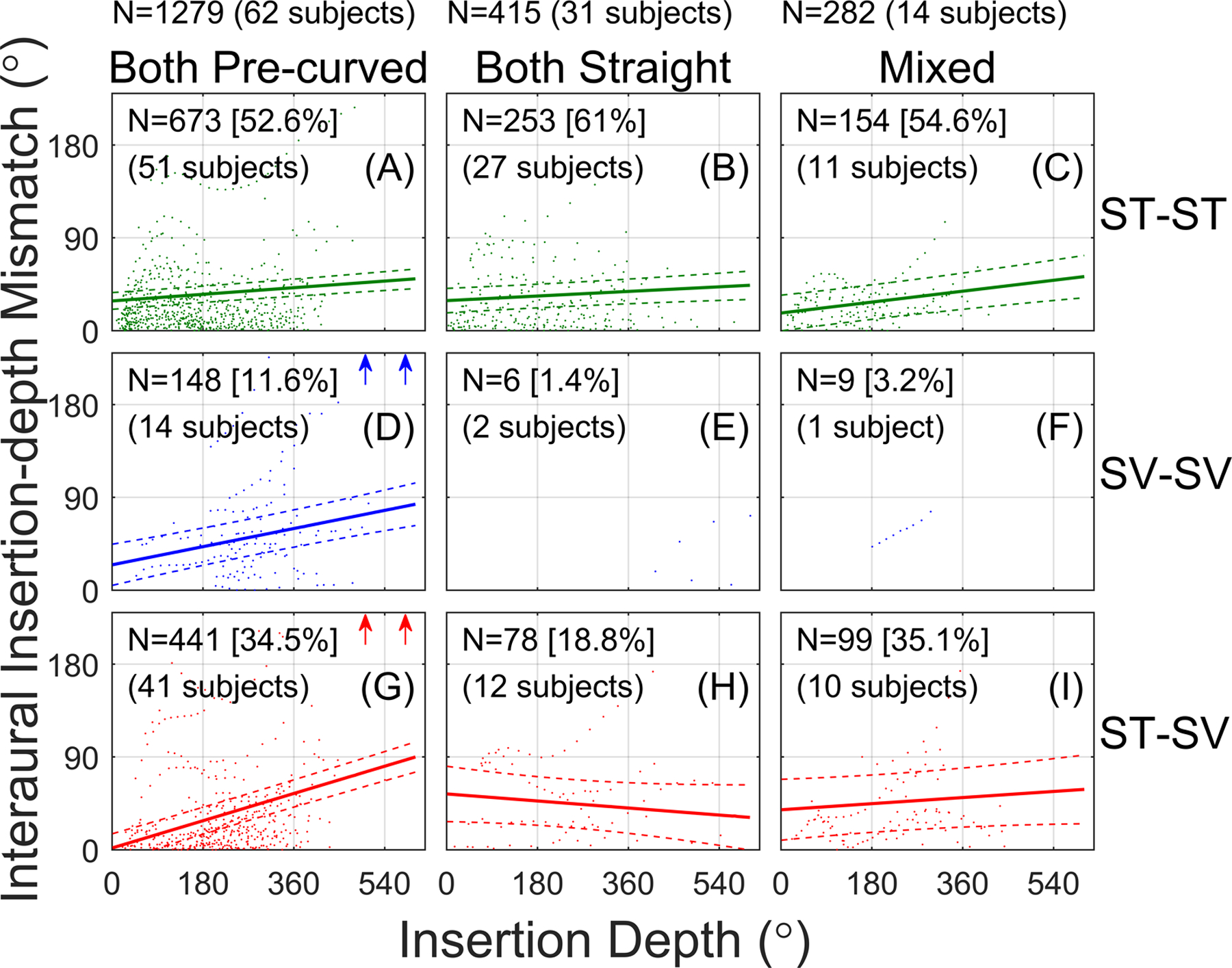
Interaural insertion-depth mismatch as a function of the average insertion depth for a given electrode pair. Each panel represents a different combination of scalar location (rows) and array type (columns). Solid lines show linear fits to the data from the linear mixed effects model. Dashed lines show the 95% confidence interval of the fits. The number of electrode pairs (the data points) and the number of subjects contributing to each condition are reported in each panel. Arrows indicate cases with >240° interaural insertion-depth mismatch that fell outside the plot range to better show the fits.
The results of the linear-mixed effects analysis are reported in Table I. There were significant main effects of insertion depth (more angular mismatch for more apically located electrodes) and scalar location (ST-SV pairs showed more angular mismatch than ST-ST pairs) on interaural insertion-depth mismatch. There were, however, many significant two- and three-way interactions, suggesting that the relationship between interaural insertion-depth mismatch and insertion depth varied across array and scalar categories.
Table I:
Results of the linear mixed-effects model.
| Fixed Effects | Estimate | Std. Err. | df | t | p |
|---|---|---|---|---|---|
| Intercept | 28.74 | 4.14 | 111.40 | 6.94 | <0.001 |
| Insertion Depth | 0.04 | 0.01 | 1593.00 | 5.96 | <0.001 |
| Scala (SV-SV) | −4.18 | 8.58 | 29.79 | −0.49 | 0.629 |
| Scala (ST-SV) | −27.08 | 4.75 | 74.39 | −5.71 | <0.001 |
| Array (Both STR) | 0.32 | 7.33 | 112.10 | 0.04 | 0.965 |
| Array (Mixed) | −11.70 | 9.74 | 114.50 | −1.20 | 0.232 |
| Insertion Depth × Scala (SV-SV) | 0.06 | 0.02 | 1560.00 | 3.55 | <0.001 |
| Insertion Depth × Scala (ST-SV) | 0.11 | 0.01 | 1541.00 | 9.96 | <0.001 |
| Insertion Depth × Both STR | 17.39 | 56.67 | 441.80 | 0.31 | 0.759 |
| Insertion Depth × Mixed | 52.13 | 12.55 | 103.90 | 4.15 | <0.001 |
| Scala (SV-SV) × Both STR | −6.97 | 42.03 | 118.60 | −0.17 | 0.869 |
| Scala (ST-SV) × Both STR | 48.77 | 11.83 | 79.57 | 4.12 | <0.001 |
| Scala (SV-SV) × Mixed | −0.01 | 0.01 | 1585.00 | −1.02 | 0.309 |
| Scala (ST-SV) × Mixed | 0.02 | 0.02 | 1590.00 | 1.54 | 0.125 |
| Insertion Depth × Scala (SV-SV) × Both STR | −0.04 | 0.11 | 1543.00 | −0.34 | 0.733 |
| Insertion Depth × Scala (ST-SV) × Both STR | −0.17 | 0.03 | 1137.00 | −5.36 | <0.001 |
| Insertion Depth × Scala (SV-SV) × Mixed | 0.16 | 0.14 | 1548.00 | 1.13 | 0.260 |
| Insertion Depth × Scala (ST-SV) × Mixed | −0.14 | 0.03 | 1596.00 | −4.54 | <0.001 |
| Random Effects | Variane | ||||
| Subject | 942.6 | ||||
| Scala (SV-SV) | 957.4 | ||||
| Scala (ST-SV) | 546.4 | ||||
| Residual | 229.0 |
Significant effects and interactions are highlighted in bold. ST=Scala Tympani; STR=straight; SV=Scala Vestibuli.
To examine the interactions, seven post-hoc models were run (the previous analysis and six additional models with different reference levels), one for each combination of scalar location and array type, excluding the two SV-SV combinations with insufficient counts/subjects. Bonferroni corrections were applied for seven analyses (criterion: p<0.0071=0.05/7). Table II reports the slopes for the different scalar-location and array combinations. It also reports p-values for pairwise tests of the difference in the slopes of the fits, which was obtained by comparing the interactions between insertion depth and different categorical conditions (bottom four lines of Table I) for each of the re-leveled models.
Table II:
Slopes for the different scalar and array categories (left columns) and a comparison of slopes across categories (right columns).
| Slope Comparison | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slopes | ST-ST | ST-ST | ST-ST | SV-SV | SV-SV | SV-SV | ST-SV | ST-SV | ST-SV | |||||
| Scala | Array | Intercept | Slope | p | Both PC | Both STR | Mixed | Both PC | Both STR | Mixed | Both PC | Both STR | Mixed | |
| ST-ST | Both PC | 28.740 | 0.036 | <0.0001 | -- | 0.309 | 0.125 | 0.0004 | -- | -- | <0.0001 | <0.0001 | <0.0001 | |
| ST-ST | Both STR | 29.050 | 0.025 | 0.005 | -- | 0.038 | 0.733 | -- | -- | <0.0001 | 0.040 | 0.374 | ||
| ST-ST | Mixed | 17.032 | 0.059 | <0.0001 | -- | 0.260 | -- | -- | <0.0001 | 0.374 | 0.356 | |||
| SV-SV | Both PC | 24.554 | 0.098 | <0.0001 | -- | -- | -- | 0.009 | 0.205 | 0.038 | ||||
| SV-SV | Both STR | -- | -- | -- | -- | -- | -- | -- | -- | |||||
| SV-SV | Mixed | -- | -- | -- | -- | -- | -- | -- | ||||||
| ST-SV | Both PC | 1.653 | 0.147 | <0.0001 | -- | <0.0001 | <0.0001 | |||||||
| ST-SV | Both STR | 54.105 | −0.038 | 0.194 | -- | 0.061 | ||||||||
| ST-SV | Mixed | 38.721 | 0.033 | 0.173 | -- | |||||||||
Bolded p values are significant at the p<0.0071 level. PC=pre-curved; ST=Scala Tympani; STR=straight; SV=Scala Vestibuli.
Figure 4 and Table II show that interaural insertion-depth mismatch increased with increasing insertion depth for four combinations of scalar location and array type: all three scalar-location cases with both-pre-curved arrays (Fig. 4A, D, G) and the ST-ST mixed-array case (Fig. 4C). There was no significant effect of insertion depth in the ST-ST both-straight case (Fig. 4B). Here, the slope of the regression line was shallow (0.025), meaning that the interaural insertion-depth mismatch increased by only 9° for a full 360° turn around the cochlea towards the apex. There was also no effect of insertion depth in the ST-SV both-straight (Fig. 4H) and ST-SV mixed-array cases (Fig. 4I), although the confidence intervals for these cases were large because of relatively few data points.
The right half of Table II reports the pairwise comparisons of the slopes in Fig. 4. There was no significant effect of array type on the slope for the ST-ST conditions (top row of Fig. 4). There was a significant effect of array type on the slope for the ST-SV conditions (bottom row of Fig. 4), where there was a significantly greater slope for the both-pre-curved (Fig. 4G) condition compared to the both-straight (Fig. 4H) and mixed (Fig. 4I) conditions. There was a significant effect of scala location on the slope for the both pre-curved conditions (left column of Fig. 4); the slopes were significantly greater for the SV-SV (Fig. 4D) and ST-SV (Fig. 4G) conditions compared to the ST-ST condition (Fig. 4A). There were no significant changes in slope as a function of scalar type for both-straight (middle column of Fig. 4) and mixed array (right column of Fig. 4) conditions, although this might be partially due to the relatively few counts for the both-straight ST-SV (Fig. 4H) and mixed ST-SV (Fig. 4I) cases. Finally, there was a significantly greater slope for the both-pre-curved ST-SV condition (Fig. 4G) than all the other conditions, except for the both-pre-curved SV-SV condition (Fig. 4D).
To summarize, the significant three-way interaction reflected different dependencies of interaural insertion-depth mismatch on insertion depth. When both electrodes were in ST, there was a slight dependence of mismatch on insertion depth for all array-type categories (Fig. 4, top row). However, when both arrays were pre-curved, there was a greater dependence of mismatch on insertion depth when at least one of the electrodes was outside of ST (Fig. 4, left column). The largest dependence on insertion depth occurred for the cases with two pre-curved arrays and an interaural scalar mismatch (ST-SV scalar locations; Fig. 4G). For these cases, the slope was 0.147, which means that the interaural insertion-depth mismatch increased by 52.9° for a low-frequency electrode pair located one full turn toward the apex relative to a high-frequency pair.
IV. GENERAL DISCUSSION
A. Summary of Results and Relationship to Previous Studies
Many factors contribute to relatively small binaural benefits in BI-CI users, including device-related and surgical factors49–52. Interaural place-of-stimulation mismatch can reduce binaural functioning in BI-CI users because the binaural differences are computed at the level of the brainstem using frequency-matched inputs14. The prevalence of interaural place-of-stimulation mismatch systematically analyzed in a large number of patients is important to report; most psychophysical or electrophysiological studies investigating interaural place-of-stimulation mismatch have involved relatively small sample sizes (N≈10)18–21,29,30,50,53–61. Using a larger database of CT scans from N=107 BI-CI users, physical interaural place-of-stimulation mismatch was estimated based on interaural insertion-depth mismatch (insertion depth differences of individual electrode pairs, measured in degrees).
Our main finding was that interaural insertion-depth mismatch was common (Fig. 2), with a median interaural insertion-depth mismatch of 23.4° or 1.3 mm. This median value is slightly smaller than a recently reported median interaural insertion-depth mismatch of 39° in 50 children with simultaneously implanted BI-CIs, as estimated from x-rays31. More important than the magnitude of physical mismatch is the effect this would be expected to have on binaural perception. Previous reports suggest that the estimated clinically relevant range—where significant decrements in perceptual binaural sensitivity and fusion occur—includes any mismatch larger than about 3 mm17–21,29,30,41–44, which is equivalent to about 75°. In the current study, 19% of electrode pairs had >3 mm and 13% of electrode pairs had >75° of interaural insertion-depth mismatch, consistent with our hypothesis that fewer than 48% of cases would show clinically relevant mismatch.
While perceptual data are not available for the full set of subjects analyzed here, Bernstein et al.39 compared CT-scan and ITD-tuning estimates of interaural insertion-depth mismatch for up to five reference electrodes for a subset of N=19 of the BI-CI subjects from the current study. Only one subject (subject BI9) had interaural mismatch >75° based on CT-scan estimates of electrode insertion angle. For this subject, ITD sensitivity improved from chance performance (ITD discrimination threshold >5000 μs) for number-matched electrodes to thresholds of 500–1000 μs for a place-matched interaural pair (see their Fig. 2A, middle column). However, the incidence of mismatch in the estimated clinically relevant range was larger for the full set of N=107 subjects (13–19%) in the current report than for the smaller N=19 sample (5%) in Bernstein et al.39 Given the lack of perceptual data available for the relatively larger proportion of subjects who had large mismatch in the current study, the expected effect of mismatch on spatial-hearing outcomes can instead be deduced from published estimates of the shape of the binaural-sensitivity tuning curve. ITD discrimination thresholds are expected to increase by approximately a factor of two to four for a 3-mm mismatch17,30 or a factor of six for a 6-mm mismatch17.
It is important to note that the estimated impact of mismatch on binaural perception is derived from studies involving single-electrode stimulation in each ear. However, it has been suggested that BI-CI users may be less tolerant to interaural insertion-depth mismatch using multi-electrode stimulation61. There are also likely neural contributions to interaural mismatch resulting from interaurally asymmetric electrode-to-neural interfaces62,63. Finally, few data exist to relate mismatch to more ecologically relevant perceptual outcomes such as binaural speech recognition and sound localization, apart from studies that have used vocoder simulations to examine these relationships42,64–67. Given the incidence of mismatch in the BI-CI population, this is an important future direction.
Our second main finding was a significant three-way interaction between insertion depth, scala location, and array type. There was a larger interaural insertion-depth mismatch for increasing insertion depths when the electrodes had an interaural scalar mismatch and when there was at least one pre-curved array (i.e., both pre-curved or mixed arrays), but particularly when there were two pre-curved arrays (Fig. 4, Tables I and II). While this broadly confirms the second hypothesis of the study, our initial hypothesis did not include the both-pre-curved condition. Consistent with previous reports25,27,68, the probability of non-ST electrode location for a single array increased with the insertion depth in the current data set (Fig. 3). Pre-curved arrays also tended to have more mismatch (Fig. 4, Tables I and II). The instance that caused the most interaural insertion-depth mismatch was when there were two pre-curved arrays and translocation occurred, typically at the apex (Fig. 4G). Previous reports suggest that pre-curved arrays tend to undergo an initial translocation at a more basal location than straight arrays27. An early translocation likely causes a deviation in the path of the array that amplifies insertion-depth mismatch throughout the translocated portion, leading to large interaural insertion-depth mismatch with pre-curved arrays and more apical locations.
These results might account for some of the dependence of binaural sensitivity on tonotopic place that is sometimes reported in the literature6, in particular the tendency for the poorest ITD discrimination performance to occur near the apical end of the array50,51,69,70. Sensitivity might be degraded by translocations71 and interaural insertion-depth or scalar mismatch (Figs. 3 and 4), all of which become more prevalent toward the apex. These occurrences might be inconsistent across different studies with small study populations, explaining the mixed conclusions.
B. Limitations and Future Directions
While we have shown that interaural insertion-depth mismatch occurs frequently in BI-CI users, this analysis reflects only the anatomical location and does not account for other factors that might affect binaural sensitivity. Peripheral biological factors such as neural degeneration, dead regions, and tissue scarring may alter the relative populations of peripheral neurons being stimulated62,63,72. Furthermore, it is possible that binaural circuits could adapt to address interaural insertion-depth mismatch73–75, which could affect binaural functioning and/or binaural fusion44,76; however, little physiological evidence has supported such an idea to date19,39.
To assess the relationship between physical and functional mismatch more directly, future work should consider relating binaural sensitivity to CT measurements of intracochlear electrode location. Our recent measurements in a smaller group of 19 BI-CI users (a subset of the 107 BI-CI users examined here) and 23 SSD-CI listeners suggest that CT and ITD-based estimates of interaural insertion-depth mismatch are closely aligned39. In contrast, perceptual19,39,76 or electrophysiological binaural sensitivity show incongruence with pitch19. Another limitation of the current study was that even though it included >100 BI-CI users, a larger sample would have provided more statistical power to examine some of the existing subgroups (e.g., use of two straight arrays, or a mix of pre-curved and straight arrays, Fig. 4) or additional subgroups (e.g., separating out different generations of pre-curved arrays within a manufacturer), as well as accounting for the different lengths of the arrays.
C. Clinical Implications
Interaural place-of-stimulation matching has the potential to improve binaural performance in BI-CI users. During implantation, improved surgical techniques to avoid interaural insertion-depth and scalar mismatch are recommended, for example, by using CT-based surgical guidance77.
Post-implantation, knowledge of the interaural cochlear locations could guide the audiological frequency-mapping process to optimize spatial hearing. While this might be facilitated by psychophysical and electrophysiological measurements of binaural performance17–19,29,30,44, these measurements require ITD sensitivity, which is not possible for some CI users78–80, and can be laboriously time consuming30. CT scans, on the other hand, are rapid and precise, albeit with the downside of any risks associated with increased radiation exposure, especially for children81. This approach acknowledges that the binaural system requires coordination across the ears, rather than treating the two devices as separate systems.
Pre-implantation, this study also guides decision-making with respect to array selection. There are competing issues involved in the selection of an array type, including the length of the array and the intended intra-scalar location. First, one should consider that longer arrays (typically straight arrays) that might have deeper insertions would be more inclined to have interaural insertion-depth and scalar mismatch toward the apex. Second, some studies suggest that a pre-curved array might provide an advantage for monaural speech-understanding outcomes and provide better channel independence82,83, although translocations with such arrays tend to occur more basally than straight arrays27 and appear to be a potential problem linked with decreased performance71. Our results showed that pre-curved arrays were more likely than straight arrays to result in more interaural insertion-depth and scalar mismatch toward the apex. The potential consequence of interaural mismatch is reduced binaural sensitivity (although frequency-mapping programming techniques might address this issue). It is important to qualify this conclusion because the observed significant differences between array types were small and it is unclear if these differences would substantially affect perception.
ACKNOWLEDGMENTS
We thank Danielle Addington, Kelly Johnson, and Kristina DeRoy Milvae for assistance with data analysis. Portions of this work were presented at the 176th Meeting of the Acoustical Society of America, 42nd Midwinter Meeting of the Association for Research in Otolaryngology, and the 19th Conference on Implantable Auditory Prostheses.
Research reported in this publication was supported by the National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Award Numbers R01 DC015798 (J.G.W.B. and M.J.G.), R01 DC014037 (J.H.N.), and R01DC014462 (J.H.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the authors, Department of Defense, or any component agency. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to report.
References:
- 1.Peters BR, Wyss J, Manrique M. Worldwide trends in bilateral cochlear implantation. Laryngoscope. 2010;120:S17–44. [DOI] [PubMed] [Google Scholar]
- 2.Holder JT, Reynolds SM, Sunderhaus LW, Gifford RH. Current profile of adults presenting for preoperative cochlear implant evaluation. Trends Hear. 2018;22:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeder RM, Firszt JB, Holden LK, Strube MJ. A longitudinal study in adults with sequential bilateral cochlear implants: Time course for individual ear and bilateral performance. J Speech Lang Hear Res. 2014;57(3):1108–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buss E, Pillsbury HC, Buchman CA, et al. Multicenter U.S. bilateral MED-EL cochlear implantation study: Speech perception over the first year of use. Ear Hear. 2008;29(1):20–32. [DOI] [PubMed] [Google Scholar]
- 5.Litovsky RY, Parkinson A, Arcaroli J. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 2009;30(4):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litovsky RY, Parkinson A, Arcaroli J, Sammeth C. Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study. Ear Hear. 2006;27(6):714–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein JGW, Goupell MJ, Schuchman G, Rivera A, Brungart DS. Having two ears facilitates the perceptual separation of concurrent talkers for bilateral and single-sided deaf cochlear implantees. Ear Hear. 2016;37(3):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeitler DM, Dorman MF, Natale SJ, Loiselle L, Yost WA, Gifford RH. Sound source localization and speech understanding in complex listening environments by single-sided deaf listeners after cochlear implantation. Otol Neurotol. 2015;36(9):1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loizou PC, Hu Y, Litovsky R, et al. Speech recognition by bilateral cochlear implant users in a cocktail-party setting. J Acoust Soc Am. 2009;125(1):372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goupell MJ, Kan A, Litovsky RY. Spatial attention in bilateral cochlear-implant users. J Acoust Soc Am. 2016;140(3):1652–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sladen DP, Nie Y, Berg K. Investigating speech recognition and listening effort with different device configurations in adult cochlear implant users. Cochlear Implants Int. 2018;19(3):119–130. [DOI] [PubMed] [Google Scholar]
- 12.Hallberg LR, Hallberg U, Kramer SE. Self-reported hearing difficulties, communication strategies and psychological general well-being (quality of life) in patients with acquired hearing impairment. Disabil Rehabil. 2008;30(3):203–212. [DOI] [PubMed] [Google Scholar]
- 13.Kramer SE, Kapteyn TS, Festen JM. The self-reported handicapping effect of hearing disabilities. Audiology. 1998;37(5):302–312. [DOI] [PubMed] [Google Scholar]
- 14.Blanks DA, Roberts JM, Buss E, Hall JW, Fitzpatrick DC. Neural and behavioral sensitivity to interaural time differences using amplitude modulated tones with mismatched carrier frequencies. J Assoc Res Otolaryngol. 2007;8(3):393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin TC, Kuwada S, Sujaku Y. Interaural time sensitivity of high-frequency neurons in the inferior colliculus. J Acoust Soc Am. 1984;76(5):1401–1410. [DOI] [PubMed] [Google Scholar]
- 16.Yin TCT, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol. 1990;64(2):465–488. [DOI] [PubMed] [Google Scholar]
- 17.Kan A, Litovsky RY, Goupell MJ. Effects of interaural pitch-matching and auditory image centering on binaural sensitivity in cochlear-implant users. Ear Hear. 2015;36(3):e62–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kan A, Stoelb C, Litovsky RY, Goupell MJ. Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. J Acoust Soc Am. 2013;134(4):2923–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Dietz M. Comparison of interaural electrode pairing methods for bilateral cochlear implants. Trends Hear. 2015;19:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kan A, Goupell MJ, Litovsky RY. Effect of channel separation and interaural mismatch on fusion and lateralization in normal-hearing and cochlear-implant listeners. J Acoust Soc Am. 2019;146(2):1448–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goupell MJ. Interaural correlation-change discrimination in bilateral cochlear-implant users: Effects of interaural frequency mismatch, centering, and age of onset of deafness. J Acoust Soc Am. 2015;137(3):1282–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landsberger DM, Svrakic M, Roland JT Jr., Svirsky M. The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants. Ear Hear. 2015;36(5):e207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ketten DR, Skinner MW, Wang G, Vannier MW, Gates GA, Neely JG. In vivo measures of cochlear length and insertion depth of Nucleus cochlear implant electrode arrays. Ann Otol Rhinol Laryngol Suppl. 1998;175:1–16. [PubMed] [Google Scholar]
- 24.Riggs WJ, Dwyer RT, Holder JT, et al. Intracochlear electrocochleography: Influence of scalar position of the cochlear implant electrode on postinsertion results. Otol Neurotol. 2019;40(5):e503–e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risi F. Considerations and rationale for cochlear implant electrode design - Past, present and future. J Int Adv Otol. 2018;14(3):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gstoettner W, Franz P, Hamzavi J, Plenk H Jr.,Baumgartner W, Czerny C. Intracochlear position of cochlear implant electrodes. Acta oto-laryngologica. 1999;119(2):229–233. [DOI] [PubMed] [Google Scholar]
- 27.Jwair S, Prins A, Wegner I, Stokroos RJ, Versnel H, Thomeer H. Scalar translocation comparison between lateral wall and perimodiolar cochlear implant arrays - A meta-analysis. Laryngoscope. 2020;00:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadol JB, Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110(9):883–891. [DOI] [PubMed] [Google Scholar]
- 29.Long CJ, Eddington DK, Colburn HS, Rabinowitz WM. Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user. J Acoust Soc Am. 2003;114(3):1565–1574. [DOI] [PubMed] [Google Scholar]
- 30.Poon BB, Eddington DK, Noel V, Colburn HS. Sensitivity to interaural time difference with bilateral cochlear implants: Development over time and effect of interaural electrode spacing. J Acoust Soc Am. 2009;126(2):806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokolov M, Zavdy O, Raveh E, Ulanovski D, Attias Y, Hilly O. Assessment of angular insertion-depth of bilateral cochlear implants using plain x-ray scans. Otol Neurotol. 2020;41(10):1363–1368. [DOI] [PubMed] [Google Scholar]
- 32.Noble JH, Dawant BM. Automatic graph-based localization of cochlear implant electrodes in CT. Med Image Comput Comput Assist Interv. 2015;9350:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble JH, Gifford RH, Labadie RF, Dawant BM. Statistical shape model segmentation and frequency mapping of cochlear implant stimulation targets in CT. Med Image Comput Comput Assist Interv. 2012;15(Pt 2):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noble JH, Labadie RF, Dawant BM. Automatic classification of cochlear implant electrode cavity positioning. Med Image Comput Comput Assist Interv. 2018;11073:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ketterer MC, Aschendorff A, Arndt S, Beck R. Electrode array design determines scalar position, dislocation rate and angle and postoperative speech perception. Eur Arch Otorhinolaryngol. 2021:e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Chakravorti S, Labadie RF, Dawant BM, Noble JH. Automatic graph-based method for localization of cochlear implant electrode arrays in clinical CT with sub-voxel accuracy. Med Image Anal. 2019;52:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble JH, Labadie RF, Majdani O, Dawant BM. Automatic segmentation of intracochlear anatomy in conventional CT. IEEE Trans Biomed Eng. 2011;58(9):2625–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Dawant BM, Labadie RF, Noble JH. Automatic localization of closely spaced cochlear implant electrode arrays in clinical CTs. Med Phys. 2018;45(11):5030–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernstein JGW, Jensen KK, Stakhovskaya OA, et al. Interaural place-of-stimulation mismatch estimates using CT scans and binaural perception, but not pitch, are consistent in cochlear-implant users. J Neurosci. 2021;41(49):10161–10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verbist BM, Skinner MW, Cohen LT, et al. Consensus panel on a cochlear coordinate system applicable in histologic, physiologic, and radiologic studies of the human cochlea. Otol Neurotol. 2010;31(5):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goupell MJ, Stoelb C, Kan A, Litovsky RY. Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening. J Acoust Soc Am. 2013;133(4):2272–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goupell MJ, Stoelb CA, Kan A, Litovsky RY. The effect of simulated interaural frequency mismatch on speech understanding and spatial release from masking. Ear Hear. 2018;39(5):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stakhovskaya OA, Bernstein JGW, Noble JH, et al. Does electrode placement affect the interaural-time-difference acuity in bilateral cochlear-implant listeners? 176th Meeting of the Acoustical Society of America; 2018; Victoria, BC. [Google Scholar]
- 44.Reiss LAJ, Fowler JR, Hartling CL, Oh Y. Binaural pitch fusion in bilateral cochlear implant users. Ear Hear. 2018;39(2):390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stakhovskaya O, Sridhar D, Bonham BH, Leake PA. Frequency map for the human cochlear spiral ganglion: Implications for cochlear implants. J Assoc Res Otolaryngol. 2007;8(2):220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwood DD. A cochlear frequency-position function for several species−−29 years later. J Acoust Soc Am. 1990;87(6):2592–2605. [DOI] [PubMed] [Google Scholar]
- 47.Buildmer: Stepwise elimination and term reordering for mixed-effects regression. [computer program]. Version 1.7.12020.
- 48.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015(67):1–48. [Google Scholar]
- 49.Kan A, Litovsky RY. Binaural hearing with electrical stimulation. Hear Res. 2015;322:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Litovsky RY, Goupell MJ, Godar S, et al. Studies on bilateral cochlear implants at the University of Wisconsin’s Binaural Hearing and Speech Laboratory. Journal of the American Academy of Audiology. 2012;23(6):476–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laback B, Egger K, Majdak P. Perception and coding of interaural time differences with bilateral cochlear implants. Hear Res. 2015;322:138–150. [DOI] [PubMed] [Google Scholar]
- 52.Blamey P, Artieres F, Başkent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol Neurootol. 2013;18(1):36–47. [DOI] [PubMed] [Google Scholar]
- 53.Steel MM, Papsin BC, Gordon KA. Binaural fusion and listening effort in children who use bilateral cochlear implants: A psychoacoustic and pupillometric study. PLoS One. 2015;10(2):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He S, Brown CJ, Abbas PJ. Effects of stimulation level and electrode pairing on the binaural interaction component of the electrically evoked auditory brain stem response. Ear Hear. 2010;31(4):457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He S, Brown CJ, Abbas PJ. Preliminary results of the relationship between the binaural interaction component of the electrically evoked auditory brainstem response and interaural pitch comparisons in bilateral cochlear implant recipients. Ear Hear. 2012;33(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Hoesel RJM. Exploring the benefits of bilateral cochlear implants. Audiol Neuro-Otol. 2004;9(4):234–246. [DOI] [PubMed] [Google Scholar]
- 57.van Hoesel RJM. Sensitivity to binaural timing in bilateral cochlear implant users. J Acoust Soc Am. 2007;121(4):2192–2206. [DOI] [PubMed] [Google Scholar]
- 58.van Hoesel RJM, Clark GM. Psychophysical studies with two binaural cochlear implant subjects. J Acoust Soc Am. 1997;102(1):495–507. [DOI] [PubMed] [Google Scholar]
- 59.van Hoesel RJM, Tyler RS. Speech perception, localization, and lateralization with bilateral cochlear implants. J Acoust Soc Am. 2003;113(3):1617–1630. [DOI] [PubMed] [Google Scholar]
- 60.Gordon KA, Salloum C, Toor GS, van Hoesel R, Papsin BC. Binaural interactions develop in the auditory brainstem of children who are deaf: Effects of place and level of bilateral electrical stimulation. J Neurosci. 2012;32(12):4212–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williges B, Jurgens T, Hu H, Dietz M. Coherent coding of enhanced interaural cues improves sound localization in noise with bilateral cochlear implants. Trends Hear. 2018;22:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bierer JA, Faulkner KF. Identifying cochlear implant channels with poor electrode-neuron interface: Partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear. 2010;31(2):247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Long CJ, Holden TA, McClelland GH, et al. Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. J Assoc Res Otolaryngol. 2014;15(2):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staisloff HE, Lee DH, Aronoff JM. Perceptually aligning apical frequency regions leads to more binaural fusion of speech in a cochlear implant simulation. Hear Res. 2016;337:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suneel D, Staisloff H, Shayman CS, Stelmach J, Aronoff JM. Localization performance correlates with binaural fusion for interaurally mismatched vocoded speech. J Acoust Soc Am. 2017;142(3):EL276–EL280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wess JM, Brungart DS, Bernstein JGW. The effect of interaural mismatches on contralateral unmasking with single-sided vocoders. Ear Hear. 2017;38(3):374–386. [DOI] [PubMed] [Google Scholar]
- 67.Xu K, Willis S, Gopen Q, Fu QJ. Effects of spectral resolution and frequency mismatch on speech understanding and spatial release from masking in simulated bilateral cochlear implants. Ear Hear. 2020;41(5):1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morrel WG, Holder JT, Dawant BM, Noble JH, Labadie RF. Effect of scala tympani height on insertion depth of straight cochlear implant electrodes. Otolaryngol Head Neck Surg. 2020;162(5):718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Best V, Laback B, Majdak P. Binaural interference in bilateral cochlear-implant listeners. J Acoust Soc Am. 2011;130(5):2939–2950. [DOI] [PubMed] [Google Scholar]
- 70.Egger K, Majdak P, Laback B. Channel interaction and current level affect across-electrode integration of interaural time differences in bilateral cochlear-implant listeners. J Assoc Res Otolaryngol. 2016;17:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakravorti S, Noble JH, Gifford RH, et al. Further evidence of the relationship between cochlear implant electrode positioning and hearing outcomes. Otol Neurotol. 2019;40(5):617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeVries L, Scheperle R, Bierer JA. Assessing the electrode-neuron interface with the electrically evoked compound action potential, electrode position, and behavioral thresholds. J Assoc Res Otolaryngol. 2016;17(3):237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reiss LA, Turner CW, Karsten SA, Gantz BJ. Plasticity in human pitch perception induced by tonotopically mismatched electro-acoustic stimulation. Neuroscience. 2014;256:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reiss LA, Turner CW, Erenberg SR, Gantz BJ. Changes in pitch with a cochlear implant over time. J Assoc Res Otolaryngol. 2007;8(2):241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reiss LA, Ito RA, Eggleston JL, et al. Pitch adaptation patterns in bimodal cochlear implant users: Over time and after experience. Ear Hear. 2015;36(2):e23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Staisloff HE, Aronoff JM. Comparing methods for pairing electrodes across ears with cochlear implants. Ear Hear. 2021;42(5):1218–1227. [DOI] [PubMed] [Google Scholar]
- 77.Noble JH, Labadie RF, Gifford RH, Dawant BM. Image-guidance enables new methods for customizing cochlear implant stimulation strategies. IEEE Trans Neural Syst Rehabil Eng. 2013;21(5):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ehlers E, Goupell MJ, Zheng Y, Godar SP, Litovsky RY. Binaural sensitivity in children who use bilateral cochlear implants. J Acoust Soc Am. 2017;141(6):4264–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Litovsky RY, Jones GL, Agrawal S, van Hoesel R. Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans. J Acoust Soc Am. 2010;127(1):400–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salloum CA, Valero J, Wong DD, Papsin BC, van Hoesel R, Gordon KA. Lateralization of interimplant timing and level differences in children who use bilateral cochlear implants. Ear Hear. 2010;31(4):441–456. [DOI] [PubMed] [Google Scholar]
- 81.Goodman TR, Mustafa A, Rowe E. Pediatric CT radiation exposure: where we were, and where we are now. Pediatr Radiol. 2019;49(4):469–478. [DOI] [PubMed] [Google Scholar]
- 82.Holder JT, Yawn RJ, Nassiri AM, et al. Matched cohort comparison indicates superiority of precurved electrode arrays. Otol Neurotol. 2019;40(9):1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berg KA, Noble JH, Dawant BM, Dwyer RT, Labadie RF, Gifford RH. Speech recognition as a function of the number of channels in perimodiolar electrode recipients. J Acoust Soc Am. 2019;145(3):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]


