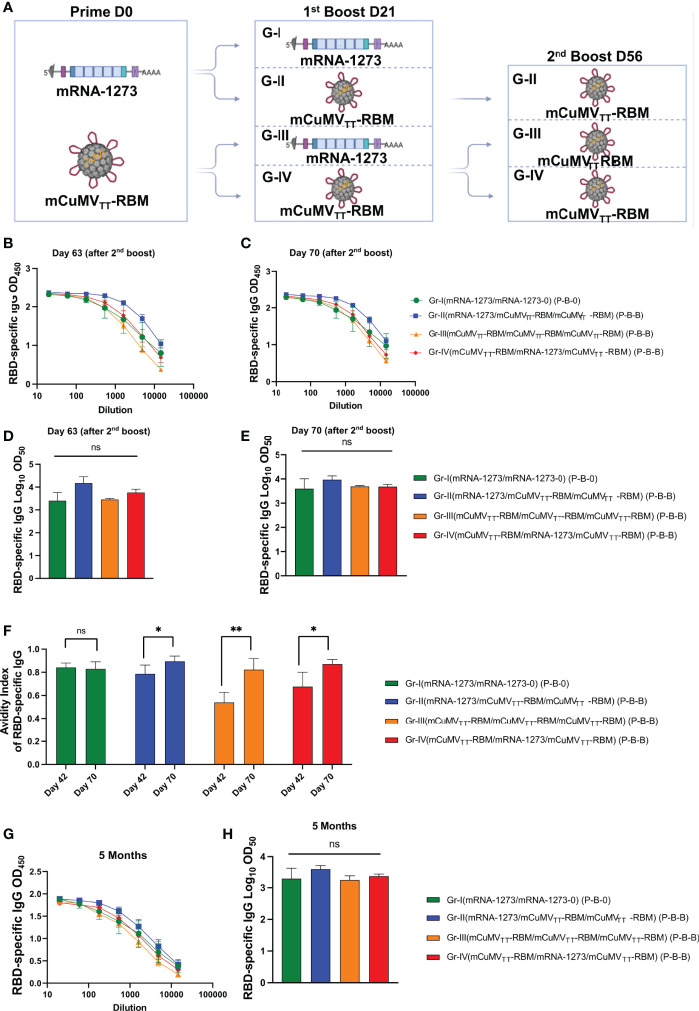
Figure 2.
Enhanced immune response with second booster dose. (A) Vaccination regimen (Prime-Boost-Boost) D0/D21/D56 and groups. Illustration was created using BioRender.com (B, C) OD450 of RBD-specific IgG for the groups vaccinated with mRNA-1273 or mCuMVTT-RBM on days 63 and 70 using D0/D21/D56 regimen. (D, E) Log10OD50 of RBD-specific IgG titers for the groups vaccinated with mRNA-1273 or mCuMVTT-RBM on 63 and 70 using D0/D21/D56 regimen. (F) Avidity Index of RBD- specific IgG in mice vaccinated with mRNA-1273 or mCuMVTT- RBM using D0/D21 or D0/D21/D56 regimens, sera were treated with PBST or 7 M Urea. (G) OD450 of RBD-specific longevity IgG for the groups vaccinated with mRNA-1273 or mCuMVTT-RBM 5 months after priming using D0/D21/D56 regimen. (H) Log10OD50 of RBD-specific IgG titers for the groups vaccinated with mRNA-1273 or mCuMVTT-RBM 5 month after priming, using D0/D21/D56 regimen. Statistical analysis (mean ± SEM) using one- way ANOVA in (D, E, H) or Student’s t test in (F), n=10 or 5. One representative of 3 similar experiments is shown. The value of *p < 0.05 was considered statistically significant (**p < 0.01). ns, statistically not significant.