Abstract
Background.
The World Health Organization, African Region, set the goal of achieving measles elimination by 2020. Namibia was one of seven African countries to implement an accelerated measles control strategy beginning in 1996. Following implementation of this strategy, measles incidence decreased; however, between 2009 and 2011 a major outbreak occurred in Namibia.
Methods.
Measles vaccination coverage data were analysed and a descriptive epidemiological analysis of the measles outbreak was conducted using measles case-based surveillance and laboratory data.
Results.
During 1989 – 2008, MCV1 (the first routine dose of measles vaccine) coverage increased from 56% to 73% and five supplementary immunisation activities were implemented. During the outbreak (August 2009 – February 2011), 4 605 suspected measles cases were reported; of these, 3 256 were confirmed by laboratory testing or epidemiological linkage. Opuwo, a largely rural district in north-western Namibia with nomadic populations, had the highest confirmed measles incidence (16 427 cases per million). Infants aged ≤11 months had the highest cumulative age-specific incidence (9 252 cases per million) and comprised 22% of all confirmed cases; however, cases occurred across a wide age range, including adults aged ≥30 years. Among confirmed cases, 85% were unvaccinated or had unknown vaccination history. The predominantly detected measles virus genotype was B3, circulating in concurrent outbreaks in southern Africa, and B2, previously detected in Angola.
Conclusion.
A large-scale measles outbreak with sustained transmission over 18 months occurred in Namibia, probably caused by importation. The wide age distribution of cases indicated measles-susceptible individuals accumulated over several decades prior to the start of the outbreak.
Measles is one of the most contagious viral diseases known and can cause severe disease and death. Measles is vaccine preventable with a safe, effective and inexpensive vaccine that has been available since 1963. The World Health Organization (WHO) currently recommends two doses of measles-containing vaccine (MCV) for all children. In Africa, routine measles vaccination for infants started in the 1980s through the Expanded Program on Immunization. Subsequently, accelerated measles control using both routine vaccination and mass campaigns started in the 1990s. Estimated measles deaths in Africa decreased by 85% from 337 000 in 2000 to 50 000 in 2010,[1] and in 2011, the goal of regional measles elimination by 2020 was set.[2]
Namibia, which gained independence from South Africa (SA) in 1990, had an estimated population of 2.1 million in 2009[3] (an annual growth rate of 2.6% was used to project a 2009 population) and a population density of 2.1 persons per square kilometre, the second-lowest population density in the world. The country is divided administratively into 34 health districts in 13 regions, including those with the highest populations in the northern part of the country along the border with Angola, and in the central and southern parts of the country. More than 67% of Namibian residents are rural dwellers.[4]
Routine measles vaccination began in Namibia in 1983, and the childhood routine immunisation schedule has included one dose of measles vaccine given to infants aged 9 months. In 1996, Namibia implemented an accelerated measles control strategy that included periodic mass vaccination campaigns. Despite these efforts, a large measles outbreak occurred during 2009 – 2011, affecting all areas of the country and people of all ages. To better understand the evolution of this outbreak and provide guidance for future measles elimination efforts, a study was conducted to describe the measles vaccination coverage through routine immunisation and supplementary immunisation activities (SIAs), and the measles epidemiology of the 2009 – 2011 outbreak.
Methods
Routine immunisation and SIAs
From 1996 to 2011, the first dose of measles-containing vaccine (MCV1) was administered at age 9 months. Additional doses of MCV were given to infants and children during periodic SIAs. In addition to the administrative MCV1 coverage data reported by the Ministry of Health and Social Services, we analysed coverage estimates for MCV1 provided by WHO/United Nations Children’s Fund (UNICEF) for children aged 1 year.[5] We calculated SIA administrative coverage by dividing the number of children vaccinated during an SIA campaign by the number of children targeted for vaccination as reported by Namibia to WHO.
Measles surveillance
We analysed annual measles cases reported using data from the WHO/UNICEF Joint Reporting Form from 1987 to 2011 and from national measles case-based surveillance data reported to the WHO from 2009 to 2011.[5] Case-based surveillance with laboratory testing for all suspected measles cases was introduced by the WHO Africa Regional Office in 1999.[6,7]
For case-based measles surveillance, the clinical case definition for suspected measles cases was an illness characterised by maculopapular rash, fever and one or more of conjunctivitis, coryza, and cough, or any illness in a patient in which the clinician suspected measles.[8] Investigations of suspected cases included collection of demographic and clinical information (i.e. age, sex, address, vaccination history and date of rash onset) and collection of a serum or urine specimen for laboratory testing. Suspected measles cases were subsequently classified as laboratory-confirmed, epidemiologically linked, clinically compatible or discarded, using the standard WHO algorithm. A suspected measles case was classified as laboratory confirmed if the case-patient had a positive measles-specific IgM antibody test and had not received measles vaccination during the 30 days before rash onset. An epidemiologically linked case was defined as one meeting the suspected measles case definition and having contact (i.e. lived in the same district or adjacent districts with plausibility of transmission) with a laboratory-confirmed measles case with rash onset during the preceding 30 days. Suspected measles cases were considered confirmed either by laboratory testing or epidemiological link. Any cases that were reported in Namibia with a date of rash onset between 2 August 2009 (epidemiological week (epi-week) 31, 2009) and 2 February 2011 (epi-week 5, 2011) were considered part of this outbreak. Incidence rates were calculated by dividing the total number of confirmed cases reported from each district by the district population and multiplying by 1 000 000. Data were analysed using Excel (Microsoft Corporation, USA) and SAS version 9.3 (SAS Institute, USA).
Laboratory diagnostics
Testing for measles-specific IgM antibody (anti-measles IgM) was performed at Namibia Institute of Pathology or the National Institute for Communicable Diseases (NICD), SA, using a standard ELISA (Enzygnost for IgM, Dade Behring, Germany). To identify circulating measles virus genotypes, molecular diagnostic testing was performed at NICD. RNA was extracted from available specimens (serum or throat swab) using the QIAamp viral RNA mini-kit (QIAGEN, Germany), and amplified by reverse transcriptase-polymerase chain reaction (RT-PCR) using primers MeV214 and MeV216, designed to target a 634-nucleotide region coding for the 3´ terminus of the nucleoprotein (N) gene (measles genotyping kit v2.0, Centers for Disease Control, USA). Amplicons were sequenced using standard protocols (BigDye Terminator v3.1 cycle sequencing kit, Applied Biosystems, USA). Measles virus genotypes were determined by phylogenetic analyses relative to the WHO measles reference strains using MEGA version 5.[9]
Results
Routine immunisation and SIAs
The WHO and UNICEF estimates of coverage with MCV1 ranged from 58 to 77% between 1991 and 2011 (Fig. 1).[5] Five SIAs were implemented in Namibia before the 2009 outbreak, in 1997, 2000, 2003, 2006 and 2009.[10,11] The SIA conducted in 2006 was subnational; the other four were nationwide. All SIAs targeted children aged 9 – 59 months, with the exception of the 1997 SIA, which targeted children aged 9 months – 14 years. Reported administrative vaccination coverage achieved during these SIAs ranged from 90 to 104%. The 2009 SIA was conducted in June, ~2 months before the measles outbreak began in Namibia. During September – December 2009, an outbreak response immunisation (ORI) SIA was conducted in the six districts most affected by the outbreak at that time (Eenhena, Engela, Okongo, Opuwo, Outapi and Rundu) (Fig. 2). The ORI SIA was selective and targeted children aged 6 – 59 months who had never been vaccinated. However, the outbreak continued and in February 2010 additional ORI activities were conducted in Opuwo district, targeting everyone aged 6 months or older irrespective of previous vaccination status. During February and March 2010, ORI campaigns were conducted in Outapi, Onandjokwe and Rundu districts, targeting children aged 6 – 59 months irrespective of previous vaccination status.
Fig. 1.
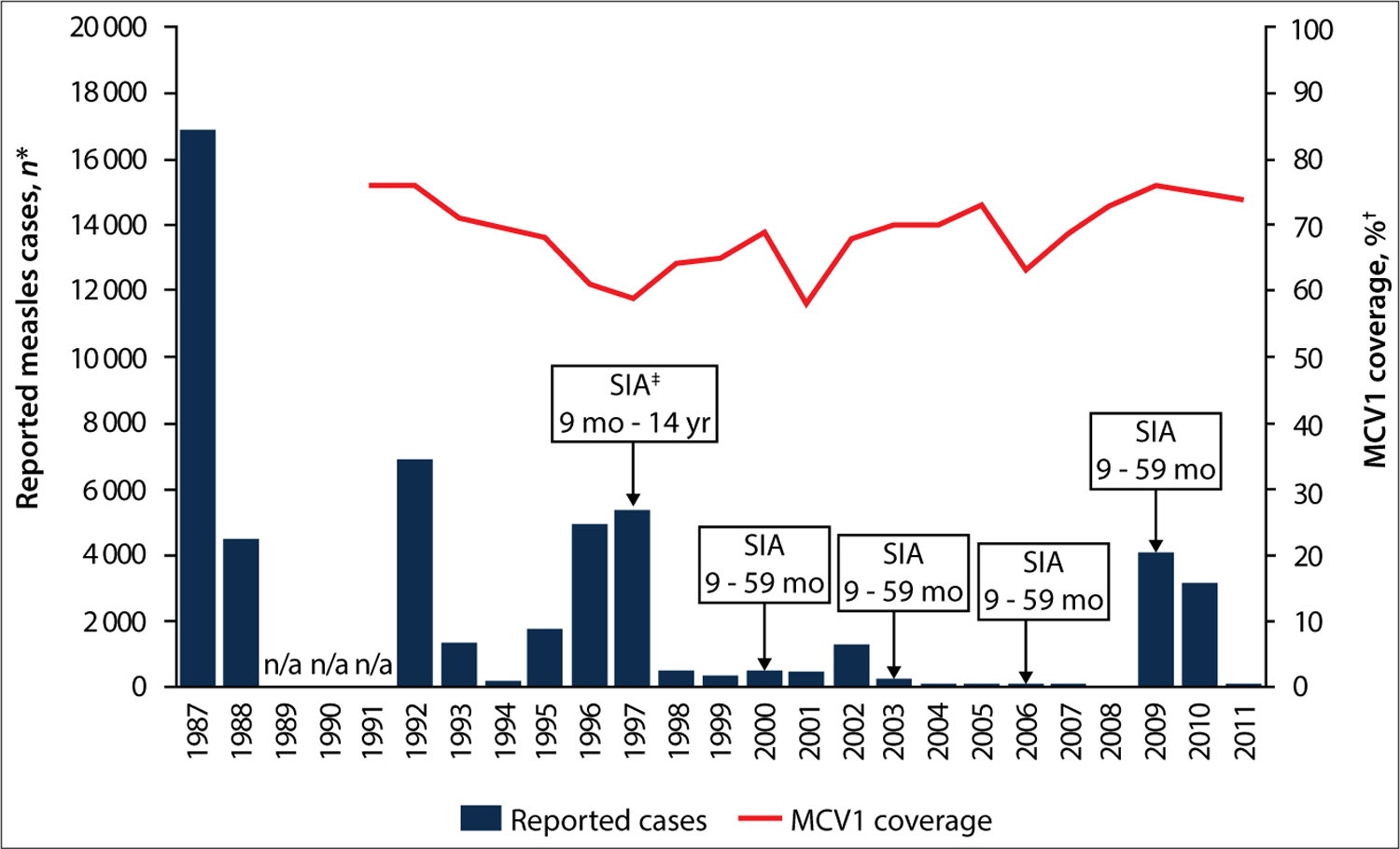
Reported measles cases, estimated coverage with MCV1 and measles SIAs, Namibia, 1987 – 2011. (n/a = data not available. *Measles cases reported annually to WHO by member states through the WHO/UNICEF JRF.[13] †WHO/UNICEF national MCV1 coverage estimates for children aged 1 year. ‡SIAs conducted in: June 1997 nationwide with administrative coverage of 92%; June 2000 nationwide with administrative coverage of 90%; June 2003 nationwide with administrative coverage of 94%; August 2006 subnational with administrative coverage of 90%; June 2009 with administrative coverage of 104%. Administrative coverage was calculated by dividing the number of measles vaccine doses administered by the targeted number of children and multiplying by 100.)
Fig. 2.
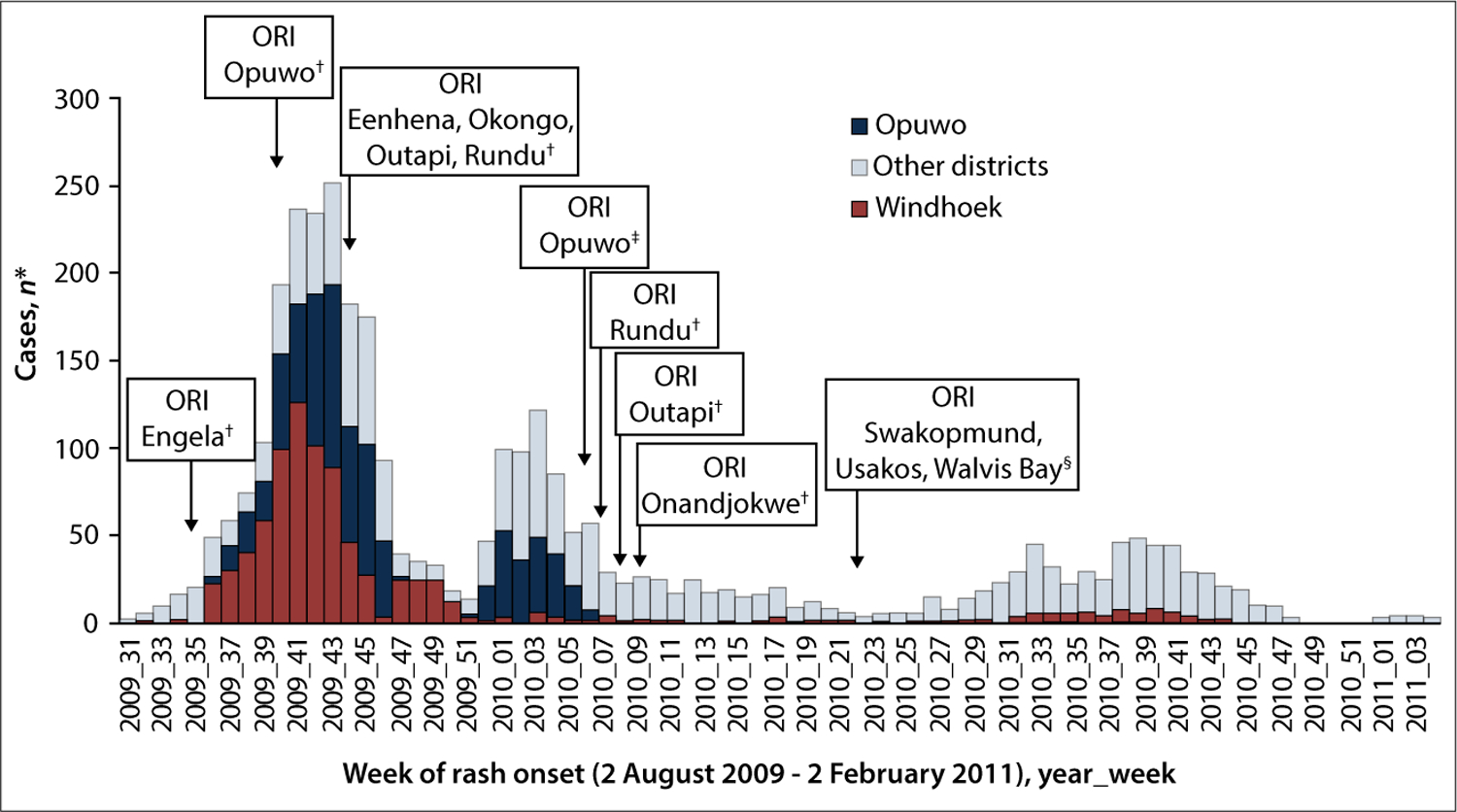
Confirmed* measles cases by epidemiological week and district, Namibia, 2 August 2009 – 2 February 2011 (N=3 256). (ORI = outbreak response immunisation. *Confirmed measles cases were defined by laboratory confirmation or epidemiological link. †Target age group: 6 – 59 months. ‡Target age group: 6 months and older. §Target age group: 6 months – 35 years.)
Measles epidemiology and outbreak response
During the outbreak (2 August 2009 – 2 February 2011), 4 605 suspected measles cases were reported in Namibia. Of these, 462 (10%) were laboratory confirmed, 2 794 (61%) were epidemiologically linked, 70 (2%) were clinically compatible but no specimen was available for testing, and 1 279 (28%) were discarded. Of the 3 256 confirmed cases, most occurred during 2009 (1 846 cases, 56.7%); 1 396 (42.9%) occurred in 2010, and 14 (0.4%) occurred in 2011 (Table 1). Thirty-two (94%) of 34 districts reported three or more confirmed cases. Only Karasburg district reported no cases during the outbreak. Twenty-seven measles-related deaths were reported.
Table 1.
Characteristics of confirmed measles cases,* Namibia, 2 August 2009 – 2 February 2011
| Namibia, n (%) | Windhoek, n (%)† | Opuwo, n (%)‡ | |
|---|---|---|---|
| Cases, N | 3 256 | 839 | 765 |
| Age group | |||
| <9 months | 495 (15.3) | 167 (20.1) | 91 (11.9) |
| 9 – 11 months | 201 (6.2) | 56 (6.7) | 45 (5.9) |
| 1 – 4 years | 519 (16.1) | 73 (8.8) | 185 (24.2) |
| 5 – 9 years | 486 (15.0) | 79 (9.5) | 110 (14.4) |
| 10 – 14 years | 305 (9.4) | 35 (4.2) | 84 (11.0) |
| ≥15 years | 1 227 (38.0) | 422 (50.7) | 249 (32.6) |
| Missing | 23 | 7 | 1 |
| Sex | |||
| Male | 1 642 (50.4) | 457 (54.5) | 370 (48.4) |
| Female | 1 614 (49.6) | 382 (45.5) | 395 (51.6) |
| Vaccination status | |||
| 0 doses | 1 025 (33.5) | 50 (6.2) | 618 (80.8) |
| 1 dose | 393 (12.8) | 53 (6.6) | 38 (5.0) |
| ≥2 doses | 71 (2.3) | 10 (1.2) | 9 (1.2) |
| Unknown | 1 570 (51.3) | 693 (86.0) | 100 (13.1) |
| Missing | 197 | 33 | |
| Year of onset | |||
| 2009 | 1 846 (56.7) | 728 (86.8) | 559 (73.1) |
| 2010 | 1 396 (42.9) | 111 (13.2) | 206 (26.9) |
| 2011 | 14 (0.4) | 0 (0.0) | 0 (0.0) |
| Classification | |||
| Laboratory confirmed | 462 (14.2) | 76 (9.1) | 25 (3.3) |
| Epi-linked | 2 794 (85.8) | 763 (90.9) | 740 (96.7) |
| Outcome | |||
| Alive | 3 226 (99.1) | 831 (99.1) | 761 (99.5) |
| Dead | 27 (0.8) | 7 (0.8) | 3 (0.4) |
| Unknown | 3 (0.1) | 1 (0.1) | 1 (0.1) |
Confirmed measles cases were defined by laboratory confirmation or epidemiological link.
Outbreak dates: 3 August 2009 – 29 October 2010.
Outbreak dates: 8 September 2009 – 8 February 2010.
Information on age and sex was available for 3 233 (99%) and 3 256 (100%) of the confirmed cases, respectively. Cases were widely distributed across all ages: 1 215 (38%) cases occurred in infants and children aged <5 years (including 696 (22%) aged ≤11 months), 791 (24%) in children aged 5 – 14 years and 1 227 (38%) in persons aged ≥15 years (Table 1 and Fig. 3). Infants aged ≤11 months had the highest cumulative incidence during the outbreak (9 252 cases per million), followed by children aged 1 – 4 years (2 306 per million) and those aged 5 – 14 years (1 468 per million) (Table 2). In urban areas, large proportions of confirmed cases were aged ≤11 months or ≥15 years (29% and 47%, respectively) relative to rural areas (19% and 39%, respectively) (Table 2).
Fig. 3.
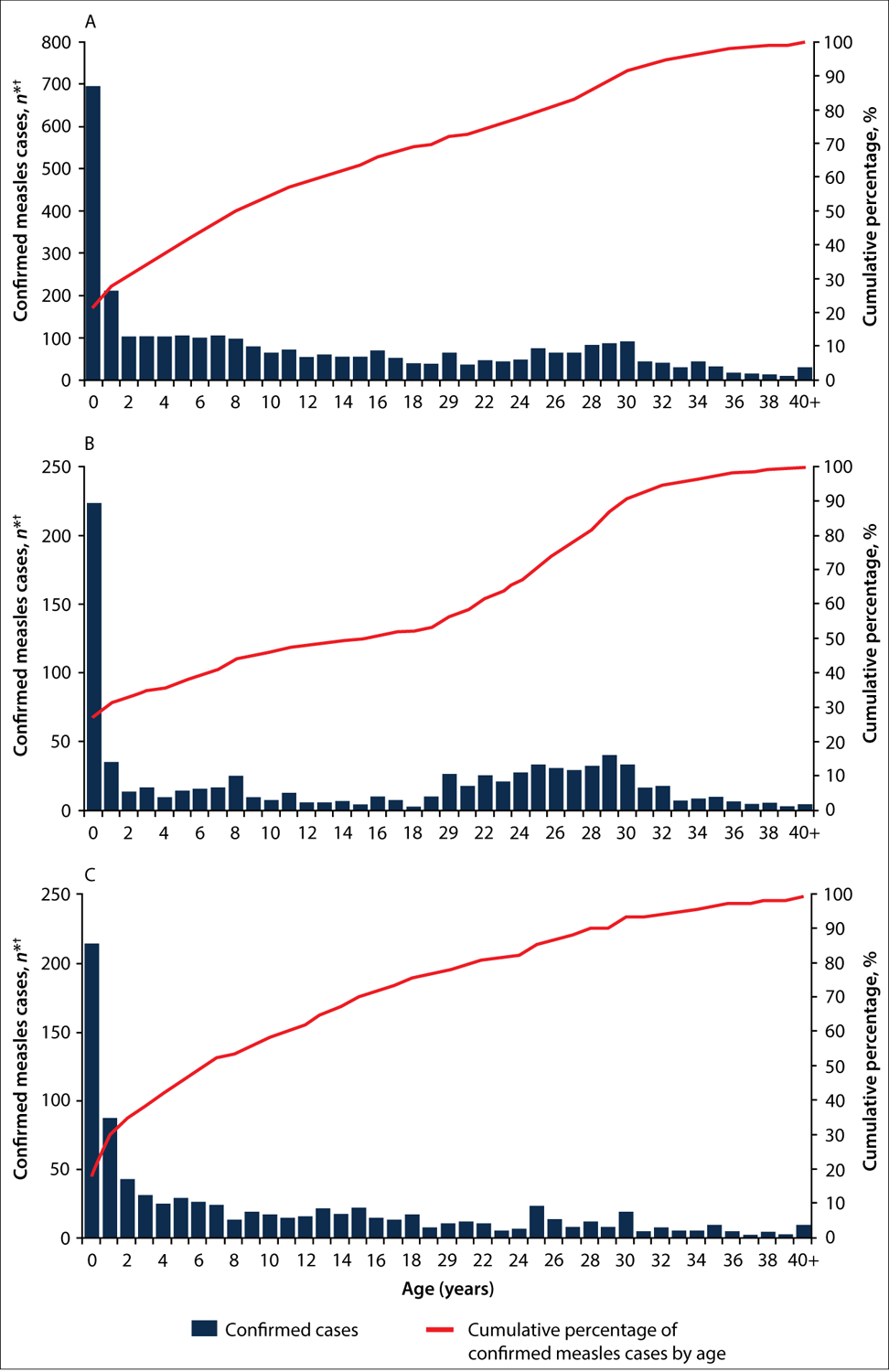
Confirmed* measles cases (N=3 233†) by age in years, Namibia, 2 August 2009 – 2 February 2011: A = Namibia; B = Windhoek; and C = Opuwo. (*Confirmed measles cases were defined by laboratory confirmation or epidemiological link. †23 confirmed cases had missing age information.)
Table 2.
Age-specific confirmed measles incidence, Namibia, 2 August 2009 – 2 February 2011
| Age group | ||||
|---|---|---|---|---|
| 0 – 11 months | 1 – 4 years | 5 – 14 years | ≥15 years | |
| Namibia (N=3 256) | ||||
| Confirmed cases, n | 696 | 519 | 791 | 1 227 |
| Population, n | 75 228 | 225 032 | 538 852 | 1 294 076 |
| Incidence, n* | 9 252 | 2 306 | 1 468 | 948 |
| Proportion of cases, % | 22 | 16 | 24 | 38 |
| Proportion of population, % | 4 | 11 | 25 | 61 |
| Windhoek† (N=839) | ||||
| Confirmed cases, n | 223 | 73 | 114 | 422 |
| Population, n | 10 823 | 25 613 | 78 343 | 177 278 |
| Incidence, n* | 20 604 | 2 850 | 1 455 | 2 380 |
| Proportion of cases, % | 27 | 9 | 14 | 51 |
| Proportion of population, % | 4 | 9 | 27 | 61 |
| Opuwo‡ (N=765) | ||||
| Confirmed cases, n | 136 | 185 | 194 | 249 |
| Population, n | 1 734 | 4 978 | 11 590 | 28 269 |
| Incidence, n* | 78 431 | 37 164 | 16 739 | 8 808 |
| Proportion of cases (%) | 18 | 24 | 25 | 33 |
| Proportion of population (%) | 4 | 11 | 25 | 61 |
| Urban§ (N=1 188) | ||||
| Confirmed cases, n | 341 | 129 | 159 | 547 |
| Proportion of cases, % | 29 | 11 | 14 | 47 |
| Rural (N=530) | ||||
| Confirmed cases, n | 98 | 83 | 141 | 203 |
| Proportion of cases, % | 19 | 16 | 27 | 39 |
Per million.
Outbreak dates: 3 August 2009 – 29 October 2010.
Outbreak dates: 8 September 2009 – 8 February 2010.
Urban/rural residence information was missing for 1 538 (47%) of confirmed cases.
Vaccination status information was available for 3 059 (95%) of confirmed cases; 1 025 (34%) were unvaccinated and 1 570 (51%) had unknown vaccination history (Table 1). The proportion of cases that had received ≥1 dose of MCV was 15% overall and low among all age groups: 11% in infants, 33% in children aged 1 – 4 years, 25% in children aged 5 – 14 years, and 3% in those aged ≥15 years. In Windhoek, 92% of confirmed cases were either unvaccinated or had unknown vaccination status, and in Opuwo 81% of confirmed cases were unvaccinated and 13% had unknown status. Before 2009, reported measles outbreaks in Namibia occurred in 1987, 1992, 1996 – 1997 and 2002 – 2003 (Fig. 1). From 2004 to 2008, fewer than 10 measles cases were reported annually. During the first half of 2009, 14 confirmed measles cases were reported; however, beginning in August 2009 (epi-week 31), clusters of cases were reported, primarily in the Engela district in northern Namibia near the border with Angola. Reported cases increased sharply in epi-week 36 and peaked with 252 cases in epi-week 43 (Fig. 2).
During the first 5 months of the outbreak (epi-weeks 31 – 52, 2009), reported measles incidence in three districts along the Angola border was >1 000 per million population (Fig. 4). Of the three districts, Opuwo had the highest confirmed incidence at 12 390 cases per million population. Opuwo has a high concentration of Ovahimba pastoralist communities, and information collected during field visits and discussions with clinicians at the Opuwo district hospital showed that most cases occurred among Ovahimba communities. Ovahimba are Bantu pastoralists who are nomadic herders of cattle and goats; although they maintain homesteads, they migrate freely throughout Kunene region on the border with Angola to find fresh vegetation to feed their livestock. Between August and December 2009, the incidence in the capital district of Windhoek was 2 500 cases per million, and a total of 22 (65%) districts had a measles incidence of ≥50 cases per million (Fig. 4).
Fig. 4.
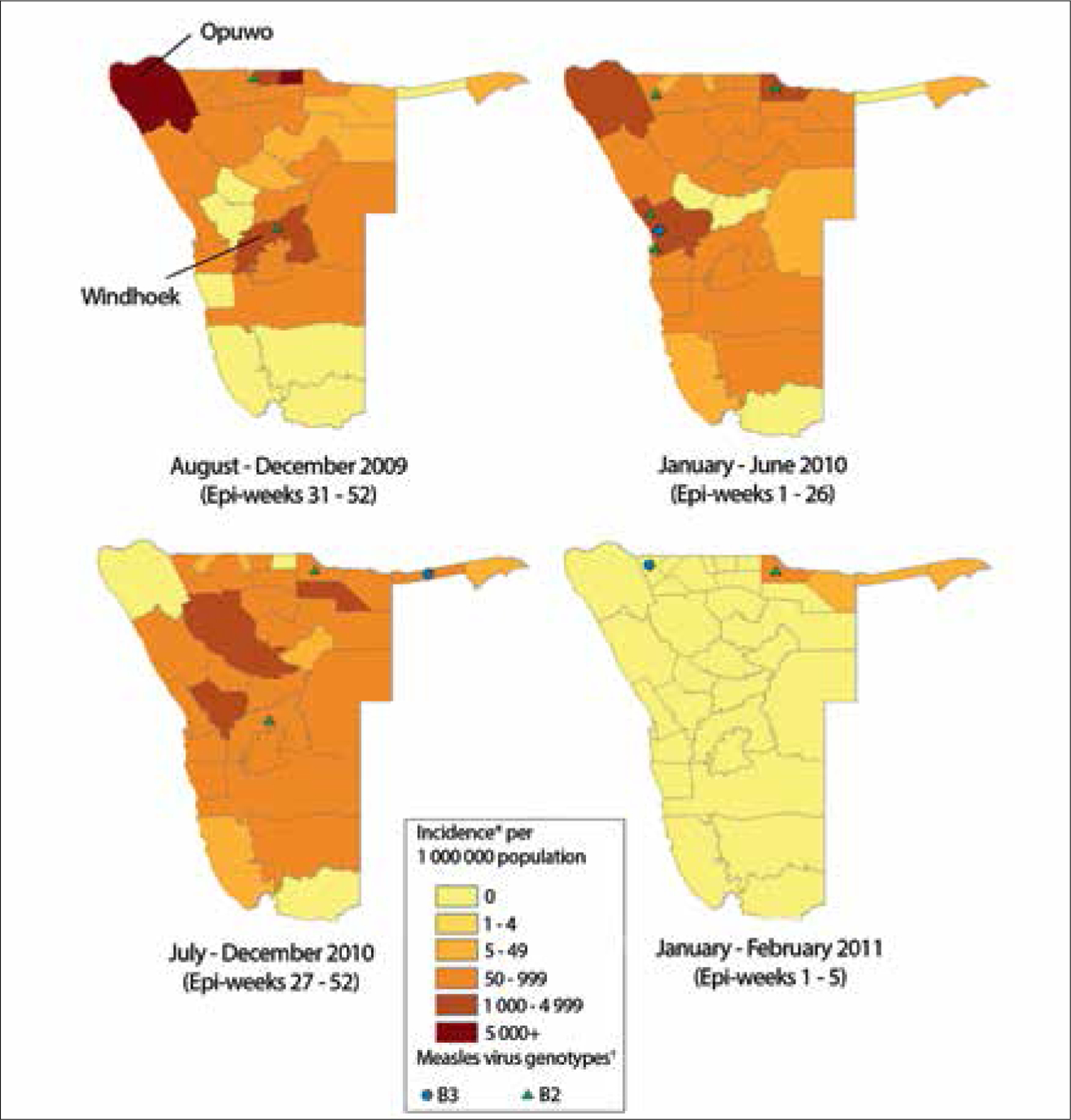
Annualised reported measles incidence* and measles virus genotype by district, Namibia, 2 August 2009 – 2 February 2011. (*Annualised measles incidence was calculated by dividing the number of confirmed measles cases from national measles case-based surveillance data in each time period by total annual population estimates from national census projections. †Each genotype symbol represents 1 – 15 specimens with that genotype detected.)
During the first half of 2010 (epi-weeks 1 – 26), four districts had measles incidence ≥1 000 cases per million, including Opuwo (3 935 cases per million) (Fig. 4). Measles incidence increased in most districts during the second half of 2010 (epi-weeks 27 – 52), but decreased to zero cases in Opuwo. Between 1 January and 2 February 2011 (end of the outbreak), only Rundu in the north-east of the country had an incidence of ≥50 cases per million population (Fig. 4). Opuwo had the highest cumulative incidence (16 427 cases per million) during the 2009 – 2011 outbreak, followed by Kongo (6 357 cases per million), Outjo (2 873 cases per million), Windhoek (2 873 cases per million), Rundu (2 304 cases per million) and Swakopmund (2 285 cases per million) districts.
Laboratory diagnostics
Genotype data were available for a total of 64 specimens from the Namibia outbreak. For August – December 2009, all four specimens from Namibia were measles virus genotype B2. During 2010, 46 (94%) of 49 specimens were genotype B2, and 3 were genotype B3 (6%). In 2011, 1 (9%) of 11 specimens was B2, and 10 (91%) were B3. Genotype B2 was previously detected in Angola and the Democratic Republic of Congo, and B3 was the predominant genotype circulating in the outbreaks in southern Africa that started in SA during 2009 – 2010.[10]
Discussion
Even with accelerated measles control efforts in Namibia beginning in 1997, MCV1 coverage estimates ranged from 59% to 77% between 1991 and 2011. However, with periodic SIAs, annual reported measles incidence decreased to less than one case per million in Namibia by 2008 and transmission of endemic measles virus was probably interrupted.[12] However, during 2009 – 2010, Namibia was one of several countries in southern Africa that experienced measles outbreaks following prolonged interepidemic periods. The outbreak in Namibia began in several districts in northern Namibia near the border with Angola; three of these districts reported measles incidence ≥1 000 cases per million, including Opuwo with the highest incidence of the outbreak of 12 390 cases per million. The likely cause of the outbreak was separate importations in 2009 and 2010 of measles virus genotypes B2 and B3. Because of an accumulation of unvaccinated, measles-susceptible individuals, these importations resulted in sustained measles virus transmission across multiple districts. The wide age distribution of cases suggests that the accumulation of measles-susceptible individuals occurred over several decades before the outbreak.
Despite modest routine measles immunisation coverage and periodic SIAs, the Namibia outbreak started 2 months after a nationwide measles SIA targeting children aged 9 – 59 months was conducted in June 2009 with 104% administrative coverage reported. Of confirmed cases during the outbreak, 22% were aged 9 – 59 months. Although most cases had missing or unknown information for vaccination status, our analysis suggested that the outbreak was due primarily to failure to achieve and sustain high two-dose measles vaccination coverage through routine immunisation services and SIAs.
The porous border between Angola and Namibia and persistently low routine measles vaccination coverage in Angola (MCV1 coverage ranged from 41% to 74% between 2000 and 2006, and from 77% to 88% between 2007 and 2009[13]) probably contributed to sustained measles virus transmission among population groups on both sides of the border. It was evident from field visits and discussions with the clinicians at the district hospital that most cases in Opuwo occurred among Ovahimba communities. Although acceptance of vaccination is high among the Ovahimba, strategies for delivering vaccination through routine services and SIAs have not adequately reached the nomadic communities. In addition, major challenges in reaching many of the rural communities in Opuwo district include the inaccessible topography (mountainous areas, impassable roads, etc.). Many hard-to-reach areas are only accessible by air.
In addition to cross-border transmission, nosocomial measles virus transmission probably played a role in sustaining the 2009 – 2011 outbreak. Only two of the five district hospitals (Opuwo and Outapi) visited during fieldwork had a separate isolation ward for measles cases. In the other outpatient units visited, there was no evidence of an isolation unit and measles cases were hospitalised in the same wards as other inpatients. In addition, measles cases among nurses were reported during the outbreak.
Several limitations of these findings should be considered. First, under-reporting of measles is well documented, so surveillance data analysis represents only a fraction of cases. Therefore, our findings are representative of reported cases and do not account for unreported outbreak cases. Second, reported administrative vaccination coverage can be biased by inaccurate estimates of population denominators and reporting of doses delivered, resulting in inaccuracies in interpreting coverage and surveillance data. Third, comparisons of annual measles case totals and incidences may be inaccurate if completeness of case reporting varies from year to year.
To achieve the goal of measles elimination by 2020, additional efforts are needed in Namibia to strengthen routine immunisation services and SIA implementation, to achieve ≥95% two-dose coverage and close immunity gaps among measles-susceptible populations. Routine immunisation services should include outreach to communities that have poor access to routine services. Targeting SIA campaigns to include older ages, similar to the ORI SIA conducted in Opuwo, could improve immunity across a wider age range. Introducing a routine second dose of measles-containing vaccine (MCV2) to the childhood immunisation schedule at age 15 – 18 months should be considered.[14,15]
The shift of measles epidemiology towards older age groups in Africa has been well documented.[7] However, follow-up SIAs have not addressed susceptibility among older age groups. SIA target age groups have been limited primarily due to inadequate resources. In April 2012, the Measles & Rubella Initiative launched the 2012 – 2020 Global Measles and Rubella Strategic Plan to support rubella with measles elimination efforts.[16] The WHO recommends that the introduction of rubella-containing vaccine should include implementation of a catch-up SIA targeted at children across a wide range of ages using combined measles and rubella vaccine.[17] In addition to contributing to rubella elimination, these SIAs would provide a unique opportunity to boost population immunity to measles and contribute to the momentum needed to achieve and sustain measles elimination. For example, when combined measles and rubella vaccine is introduced in Namibia, the initial nationwide catch-up SIA using combined measles and rubella vaccine should have a target age group that includes young adults, to close the immunity gaps that were identified during this measles outbreak.
Acknowledgements.
The authors gratefully acknowledge the work of all immunisation officers, surveillance medical officers and measles laboratory personnel across the WHO, African Region (AFR) involved in the implementation of the strategies for measles control. We also thank the outbreak investigation team for the fieldwork conducted during active case search, and the Measles & Rubella Initiative for providing financial and technical assistance to member states for strategy implementation and efforts to achieve measles elimination in AFR.
Funding.
This project was supported by the US Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1.Simons E, Ferrari M, Fricks J, et al. Assessment of the 2010 global measles mortality reduction goal: Results from a model of surveillance data. Lancet 2012;379(9832):2173–2178. DOI: 10.1016/S0140-6736(12)60522-4 [DOI] [PubMed] [Google Scholar]
- 2.Perry RT, Gacic-Dobo M, Dabbagh A, et al. Global control and regional elimination of measles, 2000 – 2012. Morb Mortal Wkly Rep 2014;63(5):103–107. [PMC free article] [PubMed] [Google Scholar]
- 3.Namibia Central Bureau of Statistics. Namibia Housing and Population Census, 2001. Namibia: NCBS, 2001. [Google Scholar]
- 4.Ministry of Health and Social Services. Namibia Demographic and Health Survey 2006 – 2007. Windhoek: MHSS, 2007. http://dhsprogram.com/pubs/pdf/FR204/FR204c.pdf (accessed 25 August 2011). [Google Scholar]
- 5.World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System 2012 Global Summary. Geneva: WHO, 2012. http://apps.who.int/immunization_monitoring/globalsummary (accessed 3 June 2013) [Google Scholar]
- 6.World Health Organization Regional Office for Africa. Case-Based Measles Surveillance with Lab Confirmation. Brazzaville: WHO Regional Office for Africa, 2010. http://www.afro.who.int/en/clusters-a-programmes/ard/immunization-and-vaccines-development/topics/mes.html (accessed 3 June 2013). [Google Scholar]
- 7.Goodson JL, Masresha BG, Wannemuehler K, Uzicanin A, Cochi S. Changing epidemiology of measles in Africa. J Infect Dis 2011;204(Suppl 1):S205–214. DOI: 10.1093/infdis/jir129 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO-Recommended Standards for Surveillance of Selected Vaccine-Preventable Diseases. Geneva: WHO, 2000. [Google Scholar]
- 9.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28(10):2731–2739. DOI: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibeshi ME, Masresha BG, Smit SB, et al. Measles resurgence in southern Africa: Challenges to measles elimination. Vaccine 2014;32(16):1798–1807. DOI: 10.1016/j.vaccine.2014.01.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Immunization, Vaccines and Biologicals. Data, Statistics and Graphics. Table 7.2. Retrospective measles data on supplementary immunization activities 2000 – 2015. Geneva: WHO, 2015. http://www.who.int/immunization/monitoring_surveillance/data/en/ (accessed 8 June 2015). [Google Scholar]
- 12.World Health Organization. Monitoring progress towards measles elimination. Wkly Epidemiol Rec 2010;85(49):490–494. [PubMed] [Google Scholar]
- 13.World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System. 2014 Global Summary. Geneva: WHO, 2014. http://apps.who.int/immunization_monitoring/globalsummary (accessed 6 May 2015). [Google Scholar]
- 14.World Health Organization. Measles vaccines: WHO position paper. Wkly Epidemiol Rec 2009;84(35):349–360. [PubMed] [Google Scholar]
- 15.World Health Organization. Recommendations of the Third Meeting of the African Regional Measles Technical Advisory Group, Accra, Ghana. 2011. Geneva: WHO, 2011. [Google Scholar]
- 16.World Health Organization. Global Measles and Rubella Strategic Plan: 2012 – 2020. Geneva: WHO, 2012. [Google Scholar]
- 17.World Health Organization. Rubella vaccines: WHO position paper. Wkly Epidemiol Rec 2011;86(29):301–316. [PubMed] [Google Scholar]


