Abstract
Background
Natriuretic peptides are routinely quantified to diagnose heart failure (HF). Their concentrations are also elevated in atrial fibrillation (AF). To clarify their value in predicting future cardiovascular events, we measured natriuretic peptides in unselected patients with cardiovascular conditions and related their concentrations to AF and HF status and outcomes.
Methods and Results
Consecutive patients with cardiovascular conditions presenting to a large teaching hospital underwent clinical assessment, 7‐day ECG monitoring, and echocardiography to diagnose AF and HF. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) was centrally quantified. Based on a literature review, four NT‐proBNP groups were defined (<300, 300–999, 1000–1999, and ≥2000 pg/mL). Clinical characteristics and NT‐proBNP concentrations were related to HF hospitalization or cardiovascular death. Follow‐up data were available in 1616 of 1621 patients (99.7%) and analysis performed at 2.5 years (median age, 70 [interquartile range, 60–78] years; 40% women). HF hospitalization or cardiovascular death increased from 36 of 488 (3.2/100 person‐years) in patients with neither AF nor HF, to 55 of 354 (7.1/100 person‐years) in patients with AF only, 92 of 369 (12.1/100 person‐years) in patients with HF only, and 128 of 405 (17.7/100 person‐years) in patients with AF plus HF (P<0.001). Higher NT‐proBNP concentrations predicted the outcome in patients with AF only (C‐statistic, 0.82; 95% CI, 0.77–0.86; P <0.001) and in other phenotype groups (C‐statistic in AF plus HF, 0.66; [95% CI, 0.61–0.70]; P <0.001).
Conclusions
Elevated NT‐proBNP concentrations predict future HF events in patients with AF irrespective of the presence of HF, encouraging routine quantification of NT‐proBNP in the assessment of patients with AF.
Keywords: atrial fibrillation, cardiovascular death, cohort study, heart failure, hospitalization, N‐terminal pro‐B‐type natriuretic peptide
Subject Categories: Atrial Fibrillation, Heart Failure
Nonstandard Abbreviations and Acronyms
- ESC
European Society of Cardiology
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
Clinical Perspective
What Is New?
NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), a biomarker known to be elevated in patients with atrial fibrillation (AF), predicts future heart failure events in unselected patients with AF.
A threshold of 1000 pg/mL is useful in identifying high‐risk patients with AF, whether or not they are diagnosed with heart failure at the time of assessment.
Similar to previous findings in heart failure, even after adjusting for important confounding variables, this threshold predicts future risk of heart events in patients with AF.
What Are the Clinical Implications?
Based on our findings, quantification of NT‐proBNP should be part of the initial assessment of unselected patients with AF to improve prediction of future cardiovascular events.
Heart failure (HF) and atrial fibrillation (AF) are found in 1% to 2% (HF) and 2% to 3% (AF) of the adult population in developed countries. Their prevalence is much greater in the elderly. 1 , 2 , 3 , 4 Both conditions are major drivers of cardiovascular morbidity and mortality. 5 , 6 , 7 Up to 50% of patients with AF suffer symptomatic HF, and comorbid AF plus HF is associated with higher morbidity and mortality than either condition alone. 5 , 6 , 7
Natriuretic peptides are released by cardiomyocytes upon stretch. They inhibit the effects of the renin‐angiotensin‐aldosterone system and the sympathetic system, lead to vasodilation, and induce diuresis. 8 , 9 Deletion of the natriuretic peptide receptor in the heart or in the endothelium causes cardiovascular dysfunction. 10 , 11 , 12 Concentrations of BNP (B‐type natriuretic peptide) and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) are elevated in patients with HF. 8 , 9 , 13 Their quantification is recommended to diagnose HF in patients presenting with dyspnea 8 , 9 , 14 and more generally to establish or rule out HF. 1 , 14 , 15 Natriuretic peptide concentrations also correlate with prognosis in patients with HF, informing therapeutic decisions such as cardiac transplantation. 14 , 15
It has long been known that natriuretic peptides are also elevated in patients with supraventricular arrhythmias, 13 including in patients with AF. 16 In addition to HF and AF, several additional factors increase the concentrations of natriuretic peptides, including age, sex, kidney function, and obesity. 8 , 9 As HF is often present in patients with AF, it is unclear to what extent the association of natriuretic peptides and outcomes is driven by HF. 8 , 9 , 14 , 17 Current guidelines for the diagnosis of HF do not advocate an adjustment of the diagnostic threshold for diagnosing HF in patients with AF. 1 , 14 The European Society of Cardiology (ESC) guidelines, for example, recommend an NT‐proBNP cutoff of 125 pg/mL in the nonacute setting and 300 pg/mL in the acute setting, to preserve the sensitivity of the test. 14 There is currently no definitive cutoff value recognized for prognostication in HF. Using natriuretic peptides as a continuous variable is important to maximize the information provided by a given measured value. 8 , 9 However, studies indicate that even after adjustment for variable such as age and renal function, elevated NT‐proBNP levels >1000 pg/mL in patients with chronic HF identify patients at high risk of cardiovascular complications. 18 , 19 , 20
To clarify the prognostic role of natriuretic peptides in patients with and without AF and HF, we quantified NT‐proBNP in an unselected contemporary cohort of multimorbid patients with cardiovascular conditions. We evaluated the risk of HF hospitalization or cardiovascular death in patients with neither AF nor HF, AF only, HF only, or AF plus HF, and determined whether NT‐proBNP concentrations predict future composite outcome in each group of patients.
Methods
Study Population
The Birmingham and Black Country Atrial Fibrillation registry enrolled consecutive patients presenting to a large teaching hospital serving a population of ≈500 000 (Sandwell and West Birmingham NHS Trust) with either diagnosed AF or at least 2 cardiovascular conditions. Details have been published. 21 Exclusion criteria were age <18 years, inability to consent, and a life expectancy <1 year. Clinical information was collected from a detailed interview, review of written and electronic hospital records, and review of medical charts for each patient. Blood pressure and anthropometric measurements including weight, height, and body mass index were recorded at baseline. A 12‐lead ECG and echocardiography were performed in all patients. All patients without diagnosed AF underwent 7‐day ambulatory ECG monitoring and were subsequently reclassified if AF was detected. Patients with atrial flutter were included in the AF group. 22
AF and HF phenotypes were determined on the basis of the clinical, ECG, and imaging findings. HF was defined on the basis of established clinical parameters defined as (1) left ventricular ejection fraction of <50%, (2) a clinical diagnosis of stable HF, or (3) New York Heart Association Functional Classification class II to IV. Stable HF was defined as a preexisting diagnosis of HF on the basis of primary and secondary care records encompassing HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF). A broad definition of HF was selected for this study to include patients across the full spectrum of HF. Patients with a history of paroxysmal AF, persistent AF, permanent AF, or atrial flutter were included in the AF phenotype groups. 22 Three patients with a history of atrial high rate episodes 23 who did not fully fit into any of the phenotype groups were excluded from analysis (Figure 1).
Figure 1. Flowchart outlining patient selection and follow‐up.
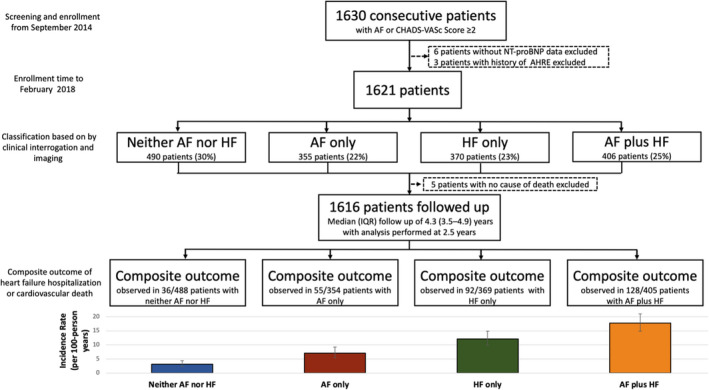
AF indicates atrial fibrillation; AHRE, atrial high rate episode; HF, heart failure; IQR, interquartile range; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Data can be made available for noncommercial use after sponsor review. Please email the corresponding author at p.kirchhof@uke.de.
Biomarker Quantification and Natriuretic Peptide Thresholds
At baseline, blood samples taken from all patients were immediately spun, fractionated, frozen, and stored at −80 °C until analysis. NT‐proBNP concentrations were quantified in a single run using commercially available Roche immunoassays (cobas Elecsys NT‐proBNP II; Roche Diagnostics, Penzberg, Germany) by personnel blinded to clinical data and outcomes. Based on a literature review, 4 NT‐proBNP concentration ranges were defined to stratify patients: <300, 300 to 999, 1000 to 1999, and ≥2000 pg/mL. An NT‐proBNP concentration <125 pg/mL provides a high negative predictive value for HF in the nonacute setting in patients with mild symptoms and underpins ESC guidelines for the diagnosis of HF. 9 However, an NT‐proBNP concentration <300 pg/mL also has high diagnostic utility, with a sensitivity of 99% and a negative predictive value of 98% for the diagnosis of HF. 14 , 24 This cutoff is also recommended in the 2016 ESC guidelines for the diagnostic workup of HF in the acute setting. 14 This study was conducted in an acute setting (ie, secondary care hospital), and an NT‐proBNP concentration 300 pg/mL was therefore selected for this study in line with ESC guidelines. The cutoff of 1000 pg/mL was selected on the basis of a number of studies demonstrating prognostic value in HF with NT‐proBNP levels above this threshold. 18 , 19 , 20 Finally, a cutoff of 2000 pg/mL was selected on the basis of evidence showing a high specificity for the diagnosis of HF at this threshold in the nonacute setting. 25
Follow‐Up and Outcome Data Collection
To obtain systematic information on cardiovascular death, HF hospitalizations, and other cardiovascular events, all patients were invited to attend a nurse‐led follow‐up appointment at 2 years. Data on the predefined major adverse cardiovascular events including HF hospitalization, hospitalization for acute coronary syndrome or myocardial infarction, and stroke were collected. In addition, hospital letters and discharge summaries were interrogated to extract further information on these outcomes. Hospital Episode Statistics data from the National Health Service database were also obtained for all patients. In addition, community general practitioner records were also reviewed to identify events not captured on hospital records. All events were cross‐checked and adjudicated by 3 of the authors (P.B., F.N., and P.K.). Mortality data were obtained from the centralized national database via National Health Service Digital including certified cause of death. The Medical Research Information Service Flagging Current Status Report, general practitioner records, and local death certificates were reviewed to determine cause of death. Death was classified as cardiovascular death on the basis of disease‐specific International Classification of Diseases codes (ICD 10). This included acute and chronic ischemic heart disease, stroke, systemic embolism, HF, and fatal arrhythmia as the immediate or underlying cause of death (Table S1). Other deaths were classified as noncardiovascular. HF hospitalization was defined as a discharge diagnosis of decompensated HF or a discharge diagnosis of HF that required inpatient treatment with intravenous diuretics. The primary outcome for this analysis was a composite of HF hospitalization and cardiovascular death censored at 2.5 years in all patients.
Ethics
This study was approved by the National Research Ethics Service Committee (Birmingham and Black Country Atrial Fibrillation registry, West Midlands, UK; Integrated Research Application System ID 97753) and sponsored by the University of Birmingham, UK. All patients provided written informed consent. This study complied with the Declaration of Helsinki.
Statistical Analysis
Patients were categorized into 4 phenotype groups for analysis, namely:
Patients who had neither AF nor HF,
Patients with AF only,
Patients with HF only, and
Patients who had AF plus HF.
To describe the clinical characteristics of the cohort, continuous variables were expressed as mean (SD) or median (interquartile range [IQR]) for normal and nonnormal distributions, respectively. Normality was tested using the Shapiro‐Wilk test. The Student t test or 1‐way ANOVA was used for continuous variables with normal distribution and 2 or >2 groups, respectively. Likewise, the Wilcoxon rank‐sum test or Kruskal‐Wallis test was used for continuous variables with nonnormal distribution and 2 or >2 groups, respectively. Categorical variables were reported as counts and percentages (n [%]), and comparisons between groups were performed using the χ2 test.
Event rates were reported per 100 person‐years of follow‐up. Kaplan‐Meier curves were created to determine the prognostic significance of each patient group on the composite outcome. The end point distributions were compared using the log‐rank test. Multivariate Cox proportional hazard regression of the composite outcome (HF hospitalization or cardiovascular death) at 2.5 years against AF and HF phenotype and predefined NT‐proBNP concentration ranges in each of these phenotype groups.
The primary analysis determined the risk of the composite outcome attributable to AF, HF, and comorbid AF and HF, in these patients. Multivariate Cox proportional hazard regression of the composite outcome (HF hospitalization or cardiovascular death) at 2.5 years against AF and HF phenotype groups was performed adjusting for confounding variables. The group with neither AF nor HF was used as a reference group. Adjustment variables were selected a priori on the basis of existing literature for their relation to cardiovascular death or HF hospitalization. 14 , 26 These variables were age, sex, race, obesity (body mass index ≥30 kg/m²), hypertension, diabetes, coronary artery disease, severe valvular heart disease, left bundle‐branch block, hyponatremia (sodium <135 mmol/L), estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration equation), medical treatment with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta blockers, diuretics (thiazide or loop diuretics), and anticoagulants (novel oral anticoagulant or vitamin K antagonist). All adjustment variables were evaluated for collinearity. Variables including urea and hemoglobin were excluded as adjustment variables because of significant collinearity with estimated glomerular filtration rate and age. Left ventricular ejection fraction was also excluded as an adjustment variable given that it was used to define phenotype groups. The proportional hazards assumption was ascertained by visual examination of log (survival) graphs to ensure parallel slopes.
The secondary analysis determined the utility of NT‐proBNP for predicting the composite outcome in the 4 phenotype groups. To evaluate the value of NT‐proBNP in predicting the composite outcome of HF hospitalization or cardiovascular death, NT‐proBNP was separately analyzed in the 4 phenotype groups controlling for known confounding variables. Kaplan‐Meier curves were constructed for each patient group stratified according to the predefined NT‐proBNP concentration ranges (<300, 300–999, 1000–1999, and ≥2000 pg/mL). Multivariate analysis of the composite outcome (HF hospitalization or cardiovascular death) at 2.5 years against NT‐proBNP concentration range groups was performed in each patient group adjusting for the clinical parameters listed above. The lowest NT‐proBNP group (<300 pg/mL) was used as a reference group. This was adjusted for confounding variables listed above.
To evaluate the impact of noncardiovascular death, competing‐risks regression based on Fine and Gray’s proportional subhazards model was performed as an additional analysis. Harrell’s C‐statistic was calculated to determine the performance of NT‐proBNP in each patient group for predicting the composite outcome. This was also performed on secondary outcomes, which were defined as the individual components of the composite outcome, and all‐cause mortality for sensitivity analysis. NT‐proBNP was used as a continuous variable in this analysis. Supplementary analysis to evaluate each cutoff by measuring discrimination (Harrell’s C‐statistic), calibration (Brier score, Bayesian information criterion, Akaike information criterion, and likelihood ratio), and reclassification (integrated discrimination improvement and net reclassification improvement) were derived using an NT‐proBNP cutoff of 300 pg/mL as a reference where appropriate. The optimum concentration of NT‐proBNP to predict the composite outcome in the entire cohort and each patient group using Youden’s index was performed and evaluated with each predefined cutoff.
For multivariate analysis only, a multiple imputation technique based on a Markov chain Monte Carlo approach was used to estimate missing values for baseline body mass index, hemoglobin, and urea. 27 A 2‐sided P‐value of <0.05 was considered statistically significant. Analyses were performed using Stata version 16.1 (StataCorp, College Station, TX).
Results
A total of 1616 patients were analyzed, with a median age of 70 (IQR, 60–78) years, 40% (n=644) were female, and 77% (n=1238) were White. A total of 488 patients had neither AF nor HF, 354 patients had AF only, 369 patients had HF only, and 405 patients had AF plus HF (Table 1, Figure 1). Patients with AF plus HF were oldest, followed by patients with AF only, then patients with HF only, and finally patients with neither AF nor HF (P<0.001). Median NT‐proBNP concentration increased gradually from 215 (IQR, 71–625) pg/mL in patients with neither AF nor HF, to 607 (IQR, 217–1831) pg/mL in patients with AF only, to 889 (IQR 261–2584) pg/mL in patients with HF only, and to 1669 (IQR 607–4238) pg/mL in patients with AF plus HF (Figure 2).
Table 1.
Descriptive Baseline Statistics
| Neither AF nor HF (N=488) |
AF only (N=354) |
HF only (N=369) |
AF plus HF (N=405) |
P value across all groups | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, y, median (IQR) | 65 (56–74) | 71 (62–79) | 68 (59–77) | 74 (67–81) | <0.001 |
| Female sex, n (%) | 222/488 (45) | 150/354 (42) | 128/369 (35) | 144/405 (36) | 0.002 |
| Race, n (%) | <0.001 | ||||
| White | 332/488 (68) | 302/354 (85) | 264/369 (72) | 340/405 (84) | … |
| Asian | 100/488 (20) | 31/354 (9) | 63/369 (17) | 30/405 (7) | … |
| Afro‐Caribbean | 55/488 (11) | 20/354 (6) | 42/369 (11) | 34/405 (8) | … |
| Other | 1/488 (0.2) | 1/354 (0.3) | … | 1/405 (0.3) | |
| Heart rhythm, n (%) | <0.001 | ||||
| Sinus rhythm | 488/488 (100) | … | 369/369 (100) | … | … |
| Paroxysmal AF | … | 195/354 (55) | … | 184/405 (45) | … |
| Persistent AF | … | 76/354 (21) | … | 100/405 (25) | … |
| Permanent AF | … | 69/354 (19) | … | 102/405 (25) | … |
| Atrial flutter | … | 14/354 (4) | … | 19/405 (5) | … |
| BMI, kg/m², median (IQR)* | 29 (25–33) | 29 (25–33) | 28 (25–32) | 29 (25–33) | 0.640 |
| Systolic BP, mm Hg, median, (IQR) | 127 (113–140) | 129 (117–143) | 122 (110–136) | 121 (109–138) | <0.001 |
| Heart rate/min, median (IQR) | 68 (61–79) | 68 (58–82) | 72 (63–82) | 76 (64–90) | <0.001 |
| Ejection fraction, %, median (IQR) | 61 (57–68) | 61 (56–68) | 46 (35–58) | 46 (35–58) | <0.001 |
| Ejection fraction <50%, n (%) | … | … | 224/357 (63) | 232/388 (60) | <0.001 |
| Previous diagnosis of stable HF | … | … | 152/369 (41) | 203/405 (50) | <0.001 |
| Symptomatic HF | <0.001 | ||||
| NYHA II HF, n (%) | … | … | 143/369 (39) | 159/401 (40) | |
| NYHA III HF, n (%) | … | … | 84/369 (23) | 111/401 (28) | |
| NYHA IV HF, n (%) | … | … | 20/369 (5) | 31/401 (8) | |
| LBBB, n (%) | 6/488 (1) | 6/354 (2) | 24/369 (7) | 22/405 (5) | <0.001 |
| Medical history, n (%) | |||||
| Diabetes | 212/488 (43) | 75/354 (21) | 166/369 (45) | 112/405 (28) | <0.001 |
| Hypertension | 322/488 (66) | 205/354 (58) | 220/369 (60) | 199/405 (49) | <0.001 |
| Coronary artery disease | 224/488 (46) | 58/354 (16) | 203/369 (55) | 144/405 (36) | <0.001 |
| Hyponatremia (Na <135 mmol/L)* | 77/481 (16) | 43/327 (13) | 71/366 (19) | 57/397 (14) | 0.115 |
| Severe valvular heart disease | 9/488 (2) | 17/354 (5) | 12/369 (3) | 41/405 (10) | <0.001 |
| HF hospitalization at presentation | … | … | 23/369 (6) | 16/405 (4) | <0.001 |
| Laboratory measurements | |||||
| eGFR, mL/min per 1.73 m2, (CKD‐EPI), median (IQR) | 81 (62–94) | 73 (58–87) | 71 (52–89) | 63 (44–82) | <0.001 |
| NT‐proBNP pg/mL, median, (IQR) in entire cohort | 215 (71–625) | 607 (217–1831) | 889 (261–2584) | 1669 (607–4238) | <0.001 |
| NT‐proBNP pg/mL, median (IQR) in patients with HFpEF | … | … | 347 (108–1243) | 1051 (420–2745) | <0.001 |
| NT‐proBNP pg/mL, median, (IQR) in patients with HFrEF | … | … | 1286 (502–3642) | 2385 (961–5712) | <0.001 |
| NT‐proBNP ≥125 pg/mL, n (%) | 298/488 (61) | 295/354 (83) | 312/369 (85) | 382/405 (94) | <0.001 |
| NT‐proBNP concentration range, n (%) | <0.001 | ||||
| <300 pg/mL | 286/488 (59) | 121/354 (34) | 100/369 (27) | 56/405 (14) | … |
| 300–999 pg/mL | 107/488 (22) | 101/354 (29) | 99/369 (27) | 87/405 (21) | … |
| 1000–1999 pg/mL | 44/488 (9) | 58/354 (16) | 56/369 (15) | 79/405 (20) | … |
| ≥2000 pg/mL | 51/488 (10) | 74/354 (21) | 114/369 (31) | 183/405 (45) | … |
| Sodium, mmol/L, median (IQR)* | 138 (136–140) | 139 (137–141) | 138 (135–140) | 139 (136–141) | 0.316 |
| Urea, mmol/L, median (IQR)* | 5.5 (4.4–7.2) | 5.8 (4.8–7.4) | 6.2 (4.7–8.5) | 6.9 (5.1–10.3) | <0.001 |
| Hemoglobin, g/L, median (IQR)* | 133 (119–145) | 135 (121–146) | 129 (116–143) | 126 (112–140) | <0.001 |
| Pharmacotherapy, n (%) | |||||
| Beta blocker | 265/488 (54) | 182/354 (51) | 232/369 (63) | 229/405 (57) | 0.013 |
| ACE inhibitors or ARB | 241/488 (49) | 161/354 (45) | 215/369 (58) | 199/405 (49) | 0.005 |
| NOAC | 9/488 (2) | 158/354 (45) | 9/369 (2) | 190/405 (47) | <0.001 |
| Warfarin | 5/488 (1) | 78/354 (22) | 13/369 (4) | 110/405 (27) | <0.001 |
| Diuretic | 97/488 (20) | 90/354 (25) | 159/369 (43) | 229/405 (56) | <0.001 |
| MRA | 6/488 (1) | 9/354 (3) | 45/369 (12) | 44/405 (11) | <0.001 |
| Complex device (ICD or CRT) | 5/488 (1) | 5/354 (1) | 26/369 (7) | 38/405 (9) | <0.001 |
ACE indicates angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LBBB, left bundle‐branch block; MRA, mineralocorticoid receptor antagonist; NOAC, novel oral anticoagulant; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and NYHA, New York Heart Association.
Baseline data were missing in 3.6% of the study population for BMI, 2.4% for hemoglobin, and 2.8% for urea and sodium.
Figure 2. Boxplot showing NT‐proBNP concentrations in each patient group.
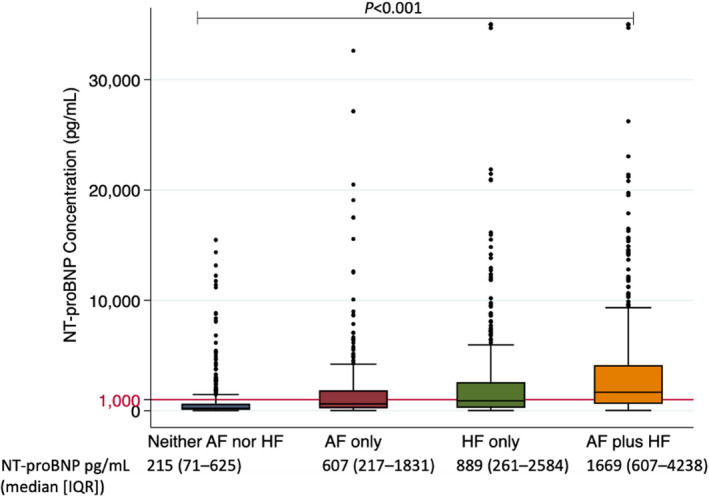
AF indicates atrial fibrillation; HF, heart failure; IQR, interquartile range; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
In 1616 of 1621 patients (99.7%), vital status and cause of death could be ascertained. It was not possible to determine the cause of death for 5 patients. These patients were excluded from the main analysis of cardiovascular death, as their cause of death could not be classified (Figure 1). The composite outcome was observed in 311 patients (19.3%) in the entire cohort (9.2/100 person‐years) with 202 HF hospitalizations and 109 cardiovascular deaths. The full baseline characteristics of each patient group according to presence or absence of the composite outcome at 2.5 years follow‐up are given in Table S2. Baseline data were missing in 3.6% of the study population for body mass index and 2.8% for sodium, and these data were imputed for multivariate analysis.
Impact of HF and AF on Outcomes
AF and HF were associated with increased risk of the composite outcome, and multivariate regression identified a graded increase in the adjusted risk for the composite outcome across the phenotype groups (Figures 3 and 4). HF hospitalization or cardiovascular death was observed in 36 (7.4%) patients with neither AF nor HF (3.2/100 person‐years), 55 (15.5%) patients with AF only (7.1/100 person‐years), 92 (24.9%) patients with HF only (12.1/100 person‐years), and 128 (31.6%) patients with AF plus HF (17.7/100 person‐years) (Figure 1, Table S3). The AF‐only phenotype remained a predictor of the composite outcome after adjustment for other variables with an adjusted hazard ratio of 2.35 (95% CI, 1.45–3.81; P=0.001). The AF‐plus‐HF phenotype was associated with the highest risk of the composite outcome, with an adjusted hazard ratio of 3.46 (95% CI, 2.20–5.46; P<0.001) (Figure 4).
Figure 3. Kaplan‐Meier curves stratified according to AF and HF phenotype groups at 2.5 years for (A) the composite outcome, (B) HF hospitalization, (C) cardiovascular death, (D) all‐cause mortality.
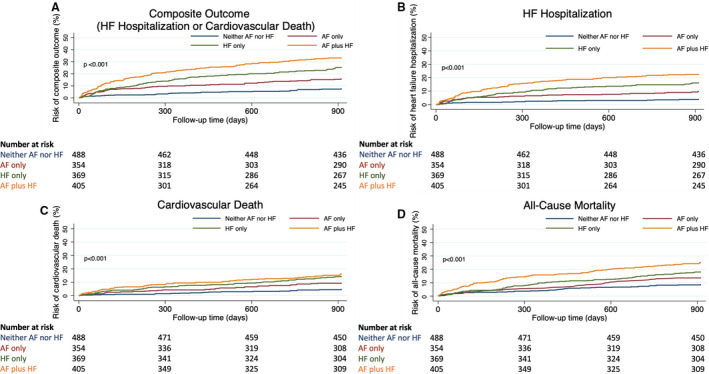
AF indicates atrial fibrillation; and HF heart failure.
Figure 4. Forest plot showing the results of multivariate Cox proportional hazards analysis for the composite outcome at 2.5 years against AF and HF phenotype groups.
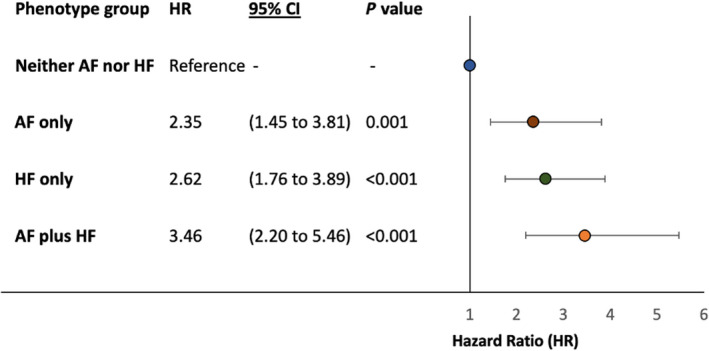
Adjusted for age, sex, race, obesity (body mass index ≥30 kg/m²), hypertension, diabetes, coronary artery disease, severe valvular heart disease, left bundle‐branch block, hyponatremia (sodium <135 mmol/L), estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration equation), medical treatment with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta blockers, diuretics (thiazide or loop diuretics), and anticoagulants (novel oral anticoagulant or vitamin K antagonist). AF indicates atrial fibrillation; and HF heart failure.
Added Information From NT‐proBNP
The NT‐proBNP concentration ranges enabled risk stratification for the composite outcome at 2.5 years’ follow‐up in the 4 phenotype groups (Figure 5). Using the NT‐proBNP <300 pg/mL concentration range as a reference group, both the NT‐proBNP 1000 to 1999 pg/mL and the NT‐proBNP ≥2000 pg/mL concentration ranges were significantly predictive of the composite outcome in the AF‐only and HF‐only phenotype groups in univariate and multivariate analyses (Table 2). In multivariate analysis, there was an incremental risk associated with higher NT‐proBNP levels in all 4 phenotype groups. In patients with HF, the increase in risk of the composite outcome reached a plateau at NT‐proBNP concentration of ≈1000 pg/mL (Figure 6). These NT‐proBNP concentration ranges also remained significantly predictive in additional competing‐risks analysis using noncardiovascular death as a competing risk (Table 2).
Figure 5. Kaplan‐Meier curves of the composite outcome at 2.5 years against baseline NT‐proBNP concentration ranges in patients with (A) neither AF nor HF, (B) AF only, (C) HF only, (D) AF plus HF.
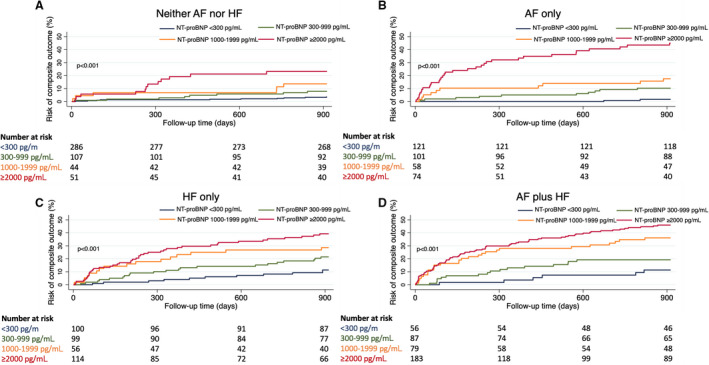
AF atrial fibrillation; HF heart failure; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Table 2.
Univariate and Multivariate Cox Proportional Hazards Analysis and Fine and Gray Regression Analysis (Noncardiovascular Death as a Competing Risk) for the Composite Outcome (HF Hospitalization or Cardiovascular Death) at 2.5 Years Against Baseline NT‐proBNP Strata in Each Patient Group
| Patient group | NT‐proBNP concentration ranges | Univariate HR (95% CI) | P value | Multivariate HR (95% CI) | P value |
Competing risks Univariate subdistribution HR (95% CI) |
P value |
Competing risks Multivariate subdistribution HR (95% CI) |
P value |
|---|---|---|---|---|---|---|---|---|---|
| Neither AF nor HF | <300 pg/mL | Reference | … | Reference | … | Reference | … | Reference | … |
| 300–999 pg/mL | 2.24 (0.88–5.67) | 0.090 | 2.07 (0.79–5.44) | 0.141 | 2.18 (0.86–5.50) | 0.099 | 1.94 (0.73–5.17) | 0.186 | |
| 1000–1999 pg/mL | 4.05 (1.47–11.15) | 0.007 | 3.71 (1.26–10.91) | 0.017 | 4.14 (1.51–11.38) | 0.006 | 3.77 (1.40–10.13) | 0.008 | |
| ≥2000 pg/mL | 7.56 (3.26–17.50) | <0.001 | 7.97 (2.87–22.10) | <0.001 | 7.72 (3.33–17.93) | <0.001 | 8.15 (3.28–20.25) | <0.001 | |
| AF only | <300 pg/mL | Reference | … | Reference | … | Reference | … | Reference | … |
| 300–999 pg/mL | 6.36 (1.40–29.05) | 0.017 | 4.15 (0.89–19.28) | 0.069 | 6.23 (1.38–28.14) | 0.017 | 3.95 (0.87–17.97) | 0.076 | |
| 1000–1999 pg/mL | 11.71 (2.56–53.43) | 0.001 | 7.99 (1.68–37.99) | 0.009 | 11.50 (2.54–52.12) | 0.002 | 7.82 (1.74–35.11) | 0.007 | |
| ≥2000 pg/mL | 37.05 (8.88–154.55) | <0.001 | 21.42 (4.77–96.18) | <0.001 | 36.27 (8.85–148.67) | <0.001 | 20.44 (4.94–84.67) | <0.001 | |
| HF only | <300 pg/mL | Reference | … | Reference | … | Reference | … | Reference | … |
| 300–999 pg/mL | 1.87 (0.92–3.81) | 0.083 | 1.44 (0.69–2.99) | 0.333 | 1.88 (0.94–3.77) | 0.074 | 1.46 (0.71–2.99) | 0.308 | |
| 1000–1999 pg/mL | 2.76 (1.31–5.83) | 0.008 | 2.28 (1.04–4.99) | 0.040 | 2.78 (1.32–5.87) | 0.007 | 2.33 (1.06–5.14) | 0.035 | |
| ≥2000 pg/mL | 3.96 (2.09–7.52) | <0.001 | 2.34 (1.10–4.97) | 0.026 | 3.87 (2.07–7.24) | <0.001 | 2.36 (1.07–5.21) | 0.034 | |
| AF plus HF | <300 pg/mL | Reference | … | Reference | … | Reference | … | Reference | … |
| 300–999 pg/mL | 1.84 (0.72–4.69) | 0.205 | 1.56 (0.60–4.04) | 0.363 | 1.82 (0.73–4.57) | 0.200 | 1.61 (0.63–4.07) | 0.317 | |
| 1000–1999 pg/mL | 3.97 (1.64–9.60) | 0.002 | 3.92 (1.59–9.71) | 0.003 | 4.02 (1.69–9.54) | 0.002 | 4.04 (1.67–9.74) | 0.002 | |
| ≥2000 pg/mL | 5.35 (2.33–12.27) | <0.001 | 4.47 (1.86–10.71) | 0.001 | 5.04 (2.25–11.32) | <0.001 | 4.30 (1.84–10.05) | 0.001 |
Multivariate analysis adjusted for age, sex, race, obesity (body mass index ≥30 kg/m²), hypertension, diabetes, coronary artery disease, hyponatremia, valvular heart disease, left bundle‐branch block, estimated glomerular filtration rate, medical treatment with angiotensin‐converting enzyme inhibitors or angiotensin receptor blocker, beta blockers, diuretics (thiazide or loop diuretics), and anticoagulants (novel oral anticoagulant or vitamin K antagonist). Baseline data were missing in 3.6% of the study population for body mass index, 2.8% for sodium and these data were imputed for multivariate analysis. AF indicates atrial fibrillation; HF, heart failure; HR, hazard ratio; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Figure 6. Forest plot showing Cox proportional hazards analysis for the composite outcome at 2.5 years against baseline NT‐proBNP concentration ranges in each patient group based on AF and HF status.
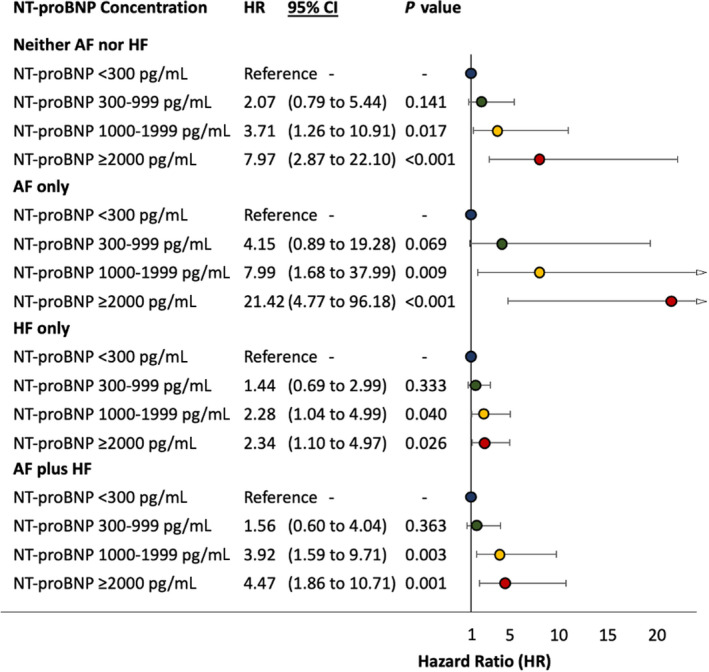
Adjusted for age, sex, race, obesity (body mass index ≥30 kg/m²), hypertension, diabetes, coronary artery disease, hyponatremia, valvular heart disease, left bundle‐branch block, estimated glomerular filtration rate, medical treatment with angiotensin‐converting enzyme inhibitors or angiotensin receptor blocker, beta blockers, diuretics (thiazide or loop diuretics), and anticoagulants (novel oral anticoagulant or vitamin K antagonist). AF indicates atrial fibrillation; HF heart failure; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Discrimination
NT‐proBNP had a higher C‐statistic for the composite outcome in the 2 phenotype groups without HF. This was 0.73 (95% CI, 0.65–0.81; P<0.001) in patients with neither AF nor HF and 0.82 (95% CI, 0.77–0.87; P<0.001) in the AF only group. Conversely, the HF‐only and AF‐plus‐HF phenotype groups had a C‐statistic of 0.66 (95% CI, 0.60–0.72; P<0.001) and 0.66 (95% CI, 0.61–0.70; P<0.001), respectively (Table S4). The impact of the presence of HFrEF compared with HFpEF on the utility of NT‐proBNP to predict the composite outcome in patients with HF only and AF plus HF was also determined as a sensitivity analysis. The C‐statistic of NT‐proBNP was similar in patients with HFrEF and HFpEF in patients with HF only and patients with AF plus HF. Ejection fraction was an important prognostic factor, but it had a limited impact on the predictive utility of NT‐proBNP. This was also the case for AF status, which had limited impact on the utility of NT‐proBNP in predicting the composite outcome in patients with HFrEF and HFpEF (Figure 7).
Figure 7. Kaplan‐Meier curves of the composite outcome at 2.5 years in (A) patients with HF only stratified according to the presence of reduced or preserved ejection fraction, (B) patients with AF plus HF stratified according to the presence of reduced or preserved ejection fraction, (C) patients with HFrEF stratified according to the presence of AF, and (D) patients with HFpEF stratified according to the presence of AF.
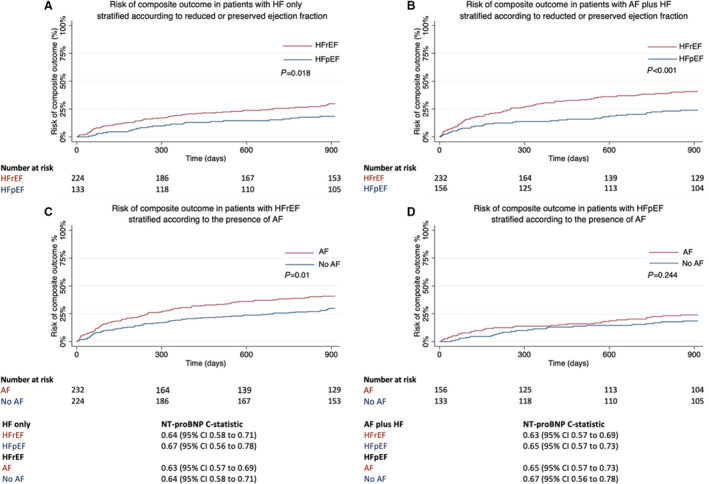
Prognosis is worse in patients with HFrEF than in those with HFpEF, and worse in those with AF than in those without AF. C‐statistic of baseline NT‐proBNP for the composite outcome in each sub‐group also shown. AF indicates atrial fibrillation; HF heart failure; HFpEF, heart failure with preserved ejection fraction; and HFrEF heart failure with reduced ejection fraction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
In terms of the predefined cutoffs, an NT‐proBNP cutoff of 1000 pg/mL performed best at discriminating the composite outcome, with a time‐to‐event analyses (Harrell’s) C‐statistic of 0.70 (0.67–0.73) in the entire cohort and 0.74 (0.68–0.80) in patients with AF only (Table S5).
Calibration
The Brier score evaluates the accuracy of probability of the best‐performing model, which is defined as the mean squared difference between the observed and predicted outcome. Brier scores range from 0 to 1.00, with 0 representing the best possible calibration. The 1000 pg/mL cutoff had the lowest Brier score when applied to the entire cohort (Table S6). The Akaike information criterion and the Bayesian information criterion were also calculated to evaluate calibration for each NT‐proBNP cutoff. The Akaike information criterion and Bayesian information criterion are both measures of the goodness of fit of a statistical model, with lower values indicating better models. The NT‐proBNP 1000 pg/mL cutoff had the lowest value when applied to the entire cohort (Table S7). The global goodness of fit of each model was also evaluated using the likelihood ratio test, with a significant P value suggesting that the newly added variable significantly improves the accuracy of the model. Relative to the NT‐proBNP 300 pg/mL cutoff, the addition of the NT‐proBNP 1000 pg/mL cutoff resulted in a statistically significant change in the likelihood ratio (Table S6).
Reclassification
Integrated discrimination improvement measures the ability of a model to improve the average sensitivity without reducing average specificity. This was performed to evaluate each NT‐proBNP cutoff relative to the NT‐proBNP 300 pg/mL cutoff as a reference. The NT‐proBNP 1000 pg/mL cutoff resulted in a statistically significant integrated discrimination improvement relative to the NT‐proBNP 300 pg/mL cutoff as a reference. Net reclassification improvement was used to evaluate the ability of each NT‐proBNP cutoff to reclassify risk. This was used to evaluate the proportion of individuals reclassified correctly relative to the NT‐proBNP 300 pg/mL cutoff as a reference. Currently, no meaningful risk categories exist for the composite outcome, and categorical net reclassification improvement was performed by nominally defining low and high risk as predicted risks of <20% and ≥20% for the composite outcome, respectively. Relative to the NT‐proBNP 300 pg/mL cutoff, the NT‐proBNP 1000 pg/mL cutoff resulted in a statistically significant reclassification in the entire cohort but this was not observed for the NT‐proBNP 2000 pg/mL cutoff (Table S6).
The optimum NT‐proBNP concentration for predicting the composite outcome in the entire cohort using Youden’s index was 1079 pg/mL (Table S5). While the optimum NT‐proBNP concentration varied across the 4 phenotype groups, an NT‐proBNP >1000 pg/mL was a significant predictor of the composite outcome in the AF and HF phenotype groups in multivariate analysis after adjusting for confounding variables. This was also the case across all 4 phenotype groups in competing‐risks analysis after adjusting for confounding variables (Table 2).
Discussion
This analysis of carefully phenotyped unselected patients with and without AF and HF diagnosed by clinical interrogation and imaging, using centrally quantified NT‐proBNP and with near‐complete 2.5‐year outcomes identified several important findings:
In unselected patients presenting to a hospital, AF is predictive of HF hospitalization or cardiovascular death in patients without clinical or echocardiographic signs of HF.
NT‐proBNP plasma concentrations improve risk stratification in patients with AF with and without HF.
Previously developed NT‐proBNP concentration thresholds can be applied to estimate risk of future cardiovascular events in patients without HF, including patients with AF (Figure 8).
Figure 8. Summary of our findings.
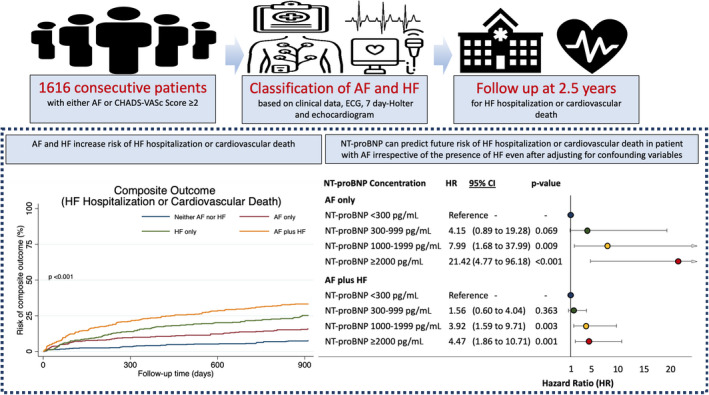
A single quantification of NT‐proBNP provides prognostic information in patients with atrial fibrillation irrespective of the presence of heart failure. AF indicates atrial fibrillation; HF heart failure; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
HF hospitalizations occur in 20% to 30% of all patients with AF, 28 reflecting that a high proportion of patients with AF suffer from heart failure, with the majority having HFpEF. 29 , 30 Our data show that patients with AF, but without an established diagnosis of heart failure, had high rates of HF hospitalization and cardiovascular death. This is important, as it highlights the need to consider adverse HF‐related outcomes in all patients with AF rather than solely in patients with HF as an established comorbidity, as currently recommended in an integrated care approach to patients with AF. 2 One important caveat to this is that in our study, we included unselected patients presenting to secondary care. More research is needed to evaluate the risk of adverse HF‐related outcomes in patients with AF but without established HF in the community or primary care setting.
The median NT‐proBNP in patients recruited to this study with AF only, that is, patients without established HF, was higher than the current ESC cutoff for diagnosing HF in the acute setting. This study therefore highlights potential limitations in the diagnostic utility of NT‐proBNP for diagnosing HF in patients with AF. Conversely, this study also highlights that NT‐proBNP has high prognostic utility in terms of predicting future HF hospitalization or cardiovascular death in patients with AF only. These results encourage the routine quantification of NT‐proBNP concentrations in the assessment of patients with AF, adding to a growing body of evidence supporting the use of this biomarker in AF for risk stratification, and elevated concentrations are associated with stroke and mortality in patients with AF. 31 Based on these findings, the use of elevated NT‐proBNP concentrations to guide screening for AF is currently being evaluated. 32
AF and atrial flutter are associated with higher concentrations of natriuretic peptides and commonly exceed the diagnostic thresholds for HF, even in the absence of further clinical evidence to support a diagnosis of HF. 9 In terms of prognosis, because of uncertainty about the prognostic significance of elevated natriuretic peptides in AF, randomized controlled trials in patients with HF have traditionally used higher natriuretic peptide thresholds in their inclusion criteria for patients with AF. Likewise, while HF risk prediction models have been developed for patients with AF, none of these models included natriuretic peptides. 33 In this study, the primary outcome of future HF hospitalization or cardiovascular death occurred more frequently in patients with AF compared with patients without AF. Even after adjusting for clinical parameters, 33 NT‐proBNP remained an important predictor of HF hospitalization or cardiovascular death in those patients with AF.
In patients with established HFrEF (left ventricular ejection fraction <35%), higher NT‐proBNP concentrations are associated with HF hospitalization or cardiovascular death, in both patients with and without AF. 34 In a similar study that included patients with HFpEF, NT‐proBNP did not predict outcomes in patients with AF as clearly. 35 This outcome may be attributable to different populations being used in different analyses. In the present study, unselected patients presenting to a hospital for cardiovascular conditions were analyzed. 35 In this setting, the risk of HF hospitalization or cardiovascular death increased at higher NT‐proBNP levels in all 4 phenotype groups. Furthermore, the association between elevated NT‐proBNP concentrations and outcomes was comparable, if not stronger, in patients with AF than in patients with HF. Treatments that are used to manage HF could explain this, such as diuretics. While diuretics reduce NT‐proBNP concentrations in patients with HF, they do not affect outcomes, suggesting that the reduction in biomarker levels attributable to diuretic therapy may be disproportionate to the associated impact on outcome. 8 , 9 , 14
Clinical Implications
This study suggests that elevated NT‐proBNP concentrations are associated with future HF hospitalization or cardiovascular death in patients with AF. This association was consistent in patients with and without clinically diagnosed HF. Hence, whenever risk prediction is clinically desired, NT‐proBNP concentrations should be measured in patients with AF. Elevated NT‐proBNP concentrations should trigger a thorough specialist evaluation, irrespective of the presence of HF.
As more disease‐modifying evidence‐based treatments become available for the management of cardiovascular disease, a major challenge for clinicians going forward will be determining what treatments to initiate for patients at risk of adverse outcomes related to HF morbidity and mortality while avoiding unnecessary treatment burden to patients and health care systems. Our data suggest that biomarkers such as natriuretic peptides, used routinely, can identify patients at risk of HF for this purpose. In line with data from previous studies in patients with HF, this study suggests that an NT‐proBNP threshold of 1000 pg/mL identifies a group of patients at high risk of future HF events.
The results of this study suggest that there is no need to adapt NT‐proBNP thresholds in patients with AF for clinical studies using HF hospitalization or cardiovascular death as an outcome, supporting previous proposals. 34 NT‐proBNP concentrations can accurately risk stratify patients with AF (with and without HF) for HF hospitalization or cardiovascular death.
In this study, the predictive utility of NT‐proBNP at discriminating end points including HF hospitalization and cardiovascular death was reduced in phenotype groups with HF compared with those without HF. The weaker association between outcomes and NT‐proBNP concentrations may be attributable to complex interactions between NT‐proBNP, the HF syndrome, and HF treatment, and may limit the effectiveness of NT‐proBNP as a prognostic marker in patients with HF. This finding warrants more research into the use of additional biomarkers to complement NT‐proBNP to help refine the prognostic assessment of patients with HF.
Limitations
This was a single‐center study that enabled comparable and comprehensive clinical phenotyping with near‐complete patient follow‐up. However, the results require external validation in different care settings. While the ceiling effect for prognostic interpretation around NT‐proBNP concentrations of <1000 pg/mL was reported before, 20 its interpretation requires caution and testing in large populations with concentrations above that threshold. A broad definition of HF was selected for this study to encompass patients across the spectrum of HF, based on current guideline recommendations including systematic imaging. While this has the important advantage of preventing patients with AF and HF being misclassified as having AF only, the use of a heterogeneous HF population and inclusion of patients with cardiomyopathy (ie, ejection fraction <50%) but potentially without established HF may increase the possibility of confounding factors in these subgroups.
Further studies are needed to elucidate if disease‐modifying evidence‐based treatments can be used to reduce the risk of future adverse events in patients with AF but without a diagnosis of HF, identified as high risk using an NT‐proBNP threshold of 1000 pg/mL. High NT‐proBNP in patients with AF may be reflective of a significant underlying atrial cardiomyopathy, and it is possible that even in the absence of symptoms, this patient group may benefit from disease‐modifying treatments to reduce the risk of future adverse events. In particular, strategies to reduce the risk of HF hospitalization in this high‐risk patient group remains a major unmet need.
Conclusions
In unselected patients presenting to a hospital, a single measurement of NT‐proBNP adds valuable prognostic information in unselected patients with AF, including patients without established HF. NT‐proBNP should be used to risk‐stratify unselected patients with AF with or without established HF. In line with previous studies on HF, an NT‐proBNP threshold of 1000 pg/mL is useful to identify high‐risk patients with AF whether or not they are diagnosed with HF at the time of assessment (Figure 8).
Sources of Funding
This study was partially supported by European Union BigData@Heart (grant agreement European Union Innovative Medicines Initiative 116074 to Dr Kirchhof) CATCH ME [Characterising Afib by Translating its Causes into Health Modifiers in the Elderly] (grant agreement ID: 633196 to Drs Kirchhof and Fabritz), AFFECT‐EU [Digital, risk‐based screening for atrial fibrillation in the European community] (grant agreement ID: 847770 to Drs Schnabel, Kirchhof, and Fabritz), and MAESTRIA [Machine Learning and Artificial intelligence for Early detection of Stroke and Atrial Fibrillation] (grant agreement ID: 965286 to Dr Fabritz at the University of Birmingham and AFNET), British Heart Foundation (FS/13/43/30324; PG/17/30/32961 and PG/20/22/35093; AA/18/2/34218), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK [German Centre for Cardiovascular Research], to Drs Kirchhof, Sshnabel, and Zeller), and Leducq Foundation to Dr Kirchhof. Dr Schnabel has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme under the grant agreement No 648131, from the European Union’s Horizon 2020 research and innovation program under the grant agreement No. 847770 (AFFECT‐EU) and German Center for Cardiovascular Research (81Z1710103); German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239).
Disclosures
Dr Fabritz has received institutional research grants for basic, translational, and clinical research projects from European Union, British Heart Foundation, Medical Research Council (UK), DFG [German Research Foundation], and from several companies active in atrial fibrillation and heart failure. Dr Kirchhof receives research support for basic, translational, and clinical research projects from the European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the past 3 years. Drs Fabritz and Kirchhof are listed as inventor on 2 patents held by the University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). Dr Schnabel has received lecture fees and advisory board fees from Bristol Myers Squibb /Pfizer outside this work.
Supporting information
Tables S1–S7
Acknowledgments
The authors thank André Ziegler and Peter Kastner, Roche Diagnostics, for quantifying NT‐proBNP concentrations in the Birmingham and Black Country Atrial Fibrillation cohort.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022833
For Sources of Funding and Disclosures, see page 14.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al.; ESC Scientific Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 4. Adderley N, Nirantharakumar K, Marshall T. Temporal variation in the diagnosis of resolved atrial fibrillation and the influence of performance targets on clinical coding: cohort study. BMJ Open. 2019;9:e030454. doi: 10.1136/bmjopen-2019-030454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nieuwlaat R, Eurlings LW, Cleland JG, Cobbe SM, Vardas PE, Capucci A, López‐Sendòn JL, Meeder JG, Pinto YM, Crijns HJGM. Atrial fibrillation and heart failure in cardiology practice: reciprocal impact and combined management from the perspective of atrial fibrillation: results of the Euro Heart Survey on atrial fibrillation. J Am Coll Cardiol. 2009;53:1690–1698. doi: 10.1016/j.jacc.2009.01.055 [DOI] [PubMed] [Google Scholar]
- 6. Chiang C‐E, Naditch‐Brûlé L, Murin J, Goethals M, Inoue H, O’Neill J, Silva‐Cardoso J, Zharinov O, Gamra H, Alam S, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real‐life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5:632–639. doi: 10.1161/CIRCEP.112.970749 [DOI] [PubMed] [Google Scholar]
- 7. Khand AU, Rankin AC, Kaye GC, Cleland JG. Systematic review of the management of atrial fibrillation in patients with heart failure. Eur Heart J. 2000;21:614–632. doi: 10.1053/euhj.1999.1767 [DOI] [PubMed] [Google Scholar]
- 8. Maisel A, Mueller C, Adams K, Anker SD, Aspromonte N, Cleland JGF, Cohen‐Solal A, Dahlstrom U, DeMaria A, Di Somma S, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. doi: 10.1016/j.ejheart.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 9. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, et al.; Heart Failure Association of the European Society of Cardiology . Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–731. doi: 10.1002/ejhf.1494 [DOI] [PubMed] [Google Scholar]
- 10. Kirchhof P, Fabritz L, Kilic A, Begrow F, Breithardt G, Kuhn M. Ventricular arrhythmias, increased cardiac calmodulin kinase II expression, and altered repolarization kinetics in ANP receptor deficient mice. J Mol Cell Cardiol. 2004;36:691–700. doi: 10.1016/j.yjmcc.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 11. Kilic A, Velic A, De Windt LJ, Fabritz L, Voss M, Mitko D, Zwiener M, Baba HA, van Eickels M, Schlatter E, et al. Enhanced activity of the myocardial Na+/H+ exchanger NHE‐1 contributes to cardiac remodeling in atrial natriuretic peptide receptor‐deficient mice. Circulation. 2005;112:2307–2317. doi: 10.1161/CIRCULATIONAHA.105.542209 [DOI] [PubMed] [Google Scholar]
- 12. Bubikat A, De Windt LJ, Zetsche B, Fabritz L, Sickler H, Eckardt D, Godecke A, Baba HA, Kuhn M. Local atrial natriuretic peptide signaling prevents hypertensive cardiac hypertrophy in endothelial nitric‐oxide synthase‐deficient mice. J Biol Chem. 2005;280:21594–21599. doi: 10.1074/jbc.M501103200 [DOI] [PubMed] [Google Scholar]
- 13. Tikkanen I, Fyhrquist F, Metsarinne K, Leidenius R. Plasma atrial natriuretic peptide in cardiac disease and during infusion in healthy volunteers. Lancet. 1985;2:66–69. doi: 10.1016/S0140-6736(85)90178-3 [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 15. Hollenberg SM, Warner Stevenson L, Ahmad T, Amin VJ, Bozkurt B, Butler J, Davis LL, Drazner MH, Kirkpatrick JN, Peterson PN, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2019;74:1966–2011. doi: 10.1016/j.jacc.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 16. Goetze JP, Friis‐Hansen L, Rehfeld JF, Nilsson B, Svendsen JH. Atrial secretion of B‐type natriuretic peptide. Eur Heart J. 2006;27:1648–1650. doi: 10.1093/eurheartj/ehl109 [DOI] [PubMed] [Google Scholar]
- 17. Knudsen CW, Omland T, Clopton P, Westheim A, Wu AHB, Duc P, McCord J, Nowak RM, Hollander JE, Storrow AB, et al. Impact of atrial fibrillation on the diagnostic performance of B‐type natriuretic peptide concentration in dyspneic patients: an analysis from the breathing not properly multinational study. J Am Coll Cardiol. 2005;46:838–844. doi: 10.1016/j.jacc.2005.05.057 [DOI] [PubMed] [Google Scholar]
- 18. Cleland JGF, McMurray JJV, Kjekshus J, Cornel JH, Dunselman P, Fonseca C, Hjalmarson Å, Korewicki J, Lindberg M, Ranjith N, et al. Plasma concentration of amino‐terminal pro‐brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin. J Am Coll Cardiol. 2009;54:1850–1859. doi: 10.1016/j.jacc.2009.06.041 [DOI] [PubMed] [Google Scholar]
- 19. Masson S, Latini R, Carbonieri E, Moretti L, Rossi MG, Ciricugno S, Milani V, Marchioli R, Struck J, Bergmann A, et al.; GISSI‐HF Investigators . The predictive value of stable precursor fragments of vasoactive peptides in patients with chronic heart failure: data from the GISSI‐heart failure (GISSI‐HF) trial. Eur J Heart Fail. 2010;12:338–347. doi: 10.1093/eurjhf/hfp206 [DOI] [PubMed] [Google Scholar]
- 20. Zile MR, Claggett BL, Prescott MF, McMurray JJV, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC, et al. Prognostic implications of changes in N‐terminal pro‐B‐type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68:2425–2436. doi: 10.1016/j.jacc.2016.09.931 [DOI] [PubMed] [Google Scholar]
- 21. Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, Thomas MR, Kotecha D, Lip GYH, Kirchhof P, et al. Data‐driven discovery and validation of circulating blood‐based biomarkers associated with prevalent atrial fibrillation. Eur Heart J. 2019;40:1268–1276. doi: 10.1093/eurheartj/ehy815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 23. Bertaglia E, Blank B, Blomström‐Lundqvist C, Brandes A, Cabanelas N, Dan G‐A, Dichtl W, Goette A, de Groot JR, Lubinski A, et al. Atrial high‐rate episodes: prevalence, stroke risk, implications for management, and clinical gaps in evidence. Europace. 2019;21:1459–1467. doi: 10.1093/europace/euz172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts E, Ludman AJ, Dworzynski K, Al‐Mohammad A, Cowie MR, McMurray JJ, Mant J. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta‐analysis in the acute care setting. BMJ. 2015;350:h910. doi: 10.1136/bmj.h910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelder JC, Cramer MJ, Verweij WM, Grobbee DE, Hoes AW. Clinical utility of three B‐type natriuretic peptide assays for the initial diagnostic assessment of new slow‐onset heart failure. J Card Fail. 2011;17:729–734. doi: 10.1016/j.cardfail.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 26. Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail. 2014;2:429–436. doi: 10.1016/j.jchf.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 27. Royston P, White IR. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Softw. 2011;2011(45):20. [Google Scholar]
- 28. Adderley NJ, Ryan R, Nirantharakumar K, Marshall T. Prevalence and treatment of atrial fibrillation in UK general practice from 2000 to 2016. Heart. 2019;105:27–33. doi: 10.1136/heartjnl-2018-312977 [DOI] [PubMed] [Google Scholar]
- 29. Nieuwlaat R, Eurlings LW, Cleland JG, Cobbe SM, Vardas PE, Capucci A, Lopez‐Sendon JL, Meeder JG, Pinto YM, Crijns HJ. Atrial fibrillation and heart failure in cardiology practice: reciprocal impact and combined management from the perspective of atrial fibrillation: results of the Euro Heart Survey on atrial fibrillation. J Am Coll Cardiol. 2009;53:1690–1698. doi: 10.1016/j.jacc.2009.01.055 [DOI] [PubMed] [Google Scholar]
- 30. Rillig A, Magnussen C, Ozga A‐K, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. 2021;144:845–858. doi: 10.1161/CIRCULATIONAHA.121.056323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, et al. N‐terminal pro‐B‐type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol. 2013;61:2274–2284. doi: 10.1016/j.jacc.2012.11.082 [DOI] [PubMed] [Google Scholar]
- 32. Engdahl J, Svennberg E, Friberg L, Al‐Khalili F, Frykman V, Kemp Gudmundsdottir K, Fredriksson T, Rosenqvist M. Stepwise mass screening for atrial fibrillation using N‐terminal pro B‐type natriuretic peptide: the STROKESTOP II study design. Europace. 2017;19:297–302. doi: 10.1093/europace/euw319 [DOI] [PubMed] [Google Scholar]
- 33. Schnabel RB, Rienstra M, Sullivan LM, Sun JX, Moser CB, Levy D, Pencina MJ, Fontes JD, Magnani JW, McManus DD, et al. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail. 2013;15:843–849. doi: 10.1093/eurjhf/hft041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kristensen SL, Jhund PS, Mogensen UM, Rørth R, Abraham WT, Desai A, Dickstein K, Rouleau JL, Zile MR, Swedberg K, et al. Prognostic value of N‐terminal pro‐B‐type natriuretic peptide levels in heart failure patients with and without atrial fibrillation. Circ: Heart Fail. 2017;10:e004409. doi: 10.1161/CIRCHEARTFAILURE.117.004409 [DOI] [PubMed] [Google Scholar]
- 35. Kristensen SL, Mogensen UM, Jhund PS, Rørth R, Anand IS, Carson PE, Desai AS, Pitt B, Pfeffer MA, Solomon SD, et al. N‐terminal pro‐B‐type natriuretic peptide levels for risk prediction in patients with heart failure and preserved ejection fraction according to atrial fibrillation status. Circ: Heart Fail. 2019;12:e005766. doi: 10.1161/CIRCHEARTFAILURE.118.005766 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7


