ABSTRACT
We report a case of a 52-year-old woman without previous underlying liver disease, presenting with progressive jaundice and diagnosed with autoimmune hepatitis after 2 doses of an inactivated coronavirus disease 2019 (CoronaVac) vaccine. All serology and histology were compatible with autoimmune hepatitis. Symptoms were improved and liver function tests were normalized after treatment with steroids and azathioprine.
INTRODUCTION
In the midst of the pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, immunization has become one of the major goals to control the contagion. The SARS-CoV-2 infection itself was associated with immune dysregulation by a potential mechanism of molecular mimicry. To date, several cases of autoimmune hepatitis (AIH) diagnosed after the administration of mRNA and viral vector COVID-19 vaccines have been reported.1–6
CASE REPORT
A 52-year-old Thai woman with hypertension and dyslipidemia received 2 doses of the CoronaVac vaccine (Sinovac Biotech, China). She was previously well until a week after receiving the second vaccine dose when she developed progressive jaundice and fatigue. Her current medications were simvastatin, amlodipine, and losartan, which she had been taking for many years. There was no history of alcohol consumption or herbal medicine usage. She denied any family history of liver disease and did not have prior results of liver function tests. Physical examination revealed a body temperature of 36.7°C with moderate icteric sclera. Abdominal examination was unremarkable. There were no stigmata of chronic liver disease. Blood chemistry showed aspartate transaminase 566 IU/L, alanine transaminase 180 IU/L, alkaline phosphatase 228 IU/L, total bilirubin 9.1 mg/dL, albumin 3.4 g/dL, globulin 4.8 g/dL, prothrombin time 14.6 seconds, international normalized ratio 1.3, and platelet count 212 × 109/L. Tests for hepatitis A, B, C, and E virus were negative. Anti-nuclear antibody and anti-smooth muscle antibody were positive at 1:160 (fine speckled) and 1:400, respectively. Anti-mitochondrial antibody was negative, and the IgG level was 2,712 mg/dL (normal: 700–1,600 mg/dL). Abdominal magnetic resonance imaging demonstrated liver cirrhosis without intrahepatic mass or bile duct dilatation. A percutaneous liver biopsy showed mixed lymphoplasmacytic infiltration with scattered lobular inflammation, interface hepatitis, and periportal bridging fibrosis (Figures 1–3). A final diagnosis of AIH cirrhosis was confirmed. She was treated with prednisolone 20 mg/day and azathioprine 75 mg/day. One month after treatment, her symptoms improved and all laboratory results normalized (aspartate transaminase 34 IU/L, alanine transaminase 14 IU/L, alkaline phosphatase 86 IU/L, and total bilirubin 0.74 mg/dL).
Figure 1.
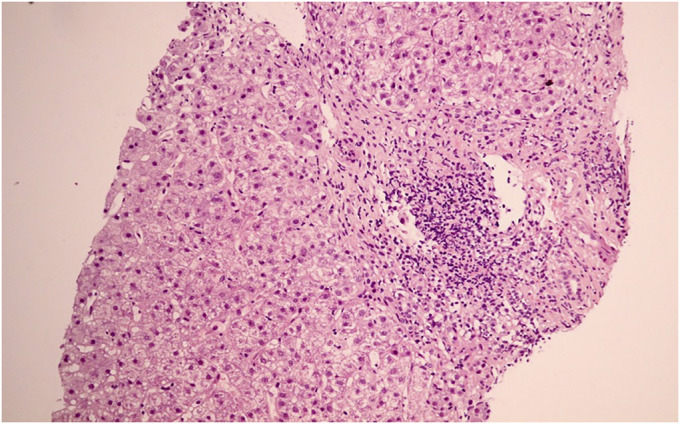
Histological staining (H&E and Masson trichrome staining). Low magnification (40×) showing portal inflammation.
Figure 3.
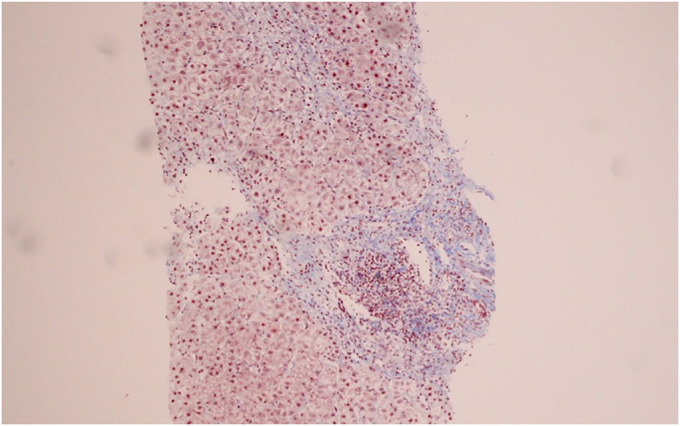
Periportal bridging fibrosis.
Figure 2.
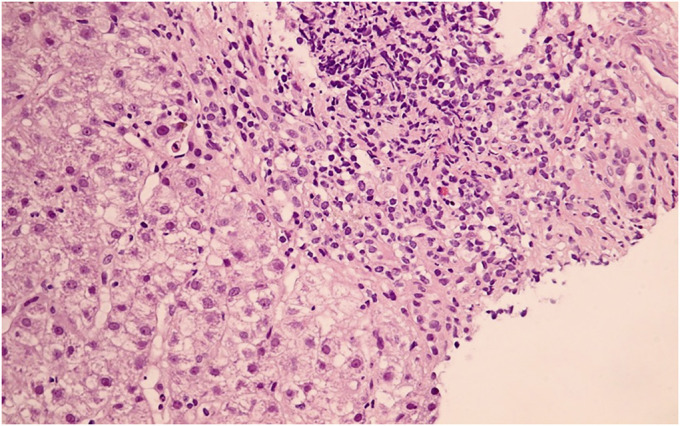
Medium magnification (200×) showing the portal tract with lymphoplasmacytic infiltration.
DISCUSSION
AIH developing after COVID-19 vaccination is still a very rare event. Only 8 cases (including our case) have been reported.1–6 Of these, 7 patients (87.5%) were women and 7 received an mRNA (Pfizer-BioNTech or Moderna) or viral vector vaccine (Covishield). To the best of our knowledge, this case is the first to develop AIH after injection of the inactivated COVID-19 vaccine. Jaundice with severe hepatitis for 1–3 weeks after vaccination was a typical presentation of all 8 reported cases. Six cases (75%) developed AIH after receiving the first dose of the vaccine. Six patients (85.7%) had positive anti-nuclear antibody, 4 (50%) had positive anti-smooth muscle antibody, and 6 had high serum IgG levels. Liver biopsy results were compatible with AIH in every case except 1 case, which had prominent eosinophils. Two patients (28.6%) had advanced fibrosis or cirrhosis from histology. Most of the patients improved after receiving prednisolone 0.5–1 mg/kg/d. However, 1 patient died after steroid therapy and plasma exchange.
Causality between the COVID-19 vaccination and the development or flare-up of AIH in these patients cannot be shown based on these 8 cases. Because these cases were reported after vaccination with different types of COVID-19 vaccines, a possible common mechanism of COVID-19 vaccine-related AIH might be an immune cross-reaction between SARS-CoV-2 proteins, especially spike protein, and human tissue proteins leading to autoantibody formation.7 Indeed, molecular mimicry and superantigen properties of SARS-CoV-2 proteins have also been offered as mechanistic explanations for autoimmune disease occurrences during natural SARS-CoV-2 infection.8,9 In addition, each type of COVID-19 vaccine promotes an immune response using different mechanisms. The mRNA vaccine uses engineered RNA to code for an antigenic protein and generates intrinsic immunogenicity. Activation of proinflammatory cascades before mRNA translation through binding of nucleotides with pattern-recognition receptors and recognition by Toll-like receptors, leading to immune activation, might play an important role in the development of immune-mediated diseases in patients receiving this type of vaccine.10 The examples of autoimmune diseases such as Guillain-Barre syndrome after the mRNA COVID-19 vaccine were reported.11,12 The viral vector vaccine (ChAdOx1nCoV-19, AstraZeneca) uses a replication-impaired chimpanzee adenoviral vector ChAdOx1 to convey the spike protein of the SARS-CoV-2 virus. There was huge sharing between the S protein and the human proteome explaining one of the mechanisms of viral vector vaccine-induced autoimmunity.13 The relationship between COVID-19 viral vector vaccine and vaccine-induced immune thrombotic thrombocytopenia by increasing antibodies to platelet factor 4 polyanion complexes was reported in several previous studies. However, the nature of these antibodies and the exact pathophysiological mechanism have not been identified.14
The inactivated vaccine stimulates immunity with a killed pathogen in combination with the adjuvants as a means to provide a safe and effective immune response. Besides possible molecular mimicry from viral components, adjuvants such as aluminum hydroxide have been linked to autoantibody induction and other autoimmune diseases in the cases of influenza virus, hepatitis B, and human papillomavirus vaccines.15 Regarding our case, AIH associated with an inactivated COVID-19 vaccine has not been found in previous studies. However, the development of AIH after receiving an inactivated hepatitis A vaccine has rarely been reported.16
In conclusion, we report the first case of AIH flare-up after receiving the second dose of an inactivated COVID-19 vaccine. We hypothesized that the COVID-19 immunization caused a provocation of an AIH flare-up because liver histology showed cirrhosis. The patient might have silent asymptomatic AIH until clinical flare-up after vaccination. Long-term monitoring of AIH events among persons receiving different vaccines is needed to clarify any potential relationship. We bring attention to this case to raise awareness to physicians about this potential postvaccine adverse event. These rare and isolated AIH cases do not affect the great benefits of receiving the COVID-19 vaccination compared with any known risks.
DISCLOSURE
Author contributions: K. Mekritthikrai and K. Thanapirom wrote the article. K. Thanapirom, P. Jaru-Ampornpan, and P. Komolmit edited the article. K. Thanapirom is the article guarantor.
Acknowledgments: We thank the research team of the Department of Medicine, Faculty of Medicine, Chulalongkorn University for English editing the final article.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Krittaya Mekritthikrai, Email: Krittaya.deer@gmail.com.
Peera Jaru-Ampornpan, Email: peera662@gmail.com.
Piyawat Komolmit, Email: piyawat.komolmit@gmail.com.
REFERENCES
- 1.Tan CK, Wong YJ, Wang LM, Ang TL, Kumar R. Autoimmune hepatitis following COVID-19 vaccination: True causality or mere association? J Hepatol. 2021;75:1250–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: May not be a causality. J Hepatol. 2021;75(3):728–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021;123:102688. [DOI] [PubMed] [Google Scholar]
- 4.Londoño MC, Gratacós-Ginès J, Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination. Still casualty? J Hepatol 2021;75:1248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty?. J Hepatol. 2021;75(1):222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McShane C, Kiat C, Rigby J, Crosbie O. The mRNA COVID-19 vaccine—a rare trigger of autoimmune hepatitis? J Hepatol. 2021;75:1252–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight JS, Caricchio R, Casanova JL, et al. The intersection of COVID-19 and autoimmunity. J Clin Invest. 2021;131(24):e154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanduc D. From anti-SARS-CoV-2 immune responses to COVID-19 via molecular mimicry. Antibodies (Basel). 2020;9(3):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2021;224:108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel SU, Khurram R, Lakhani A, Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14(4):e242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following pfizer COVID-19 vaccine. Cureus. 2021;13(2):e13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanduc D, Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol Res. 2020;68(5):310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vadalà M, Poddighe D, Laurino C, Palmieri B. Vaccination and autoimmune diseases: Is prevention of adverse health effects on the horizon? EPMA J. 2017;8(3):295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry PA, Smith-Laing G. Hepatitis A vaccine associated with autoimmune hepatitis. World J Gastroenterol. 2007;13(15):2238–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


