FIGURE 12.
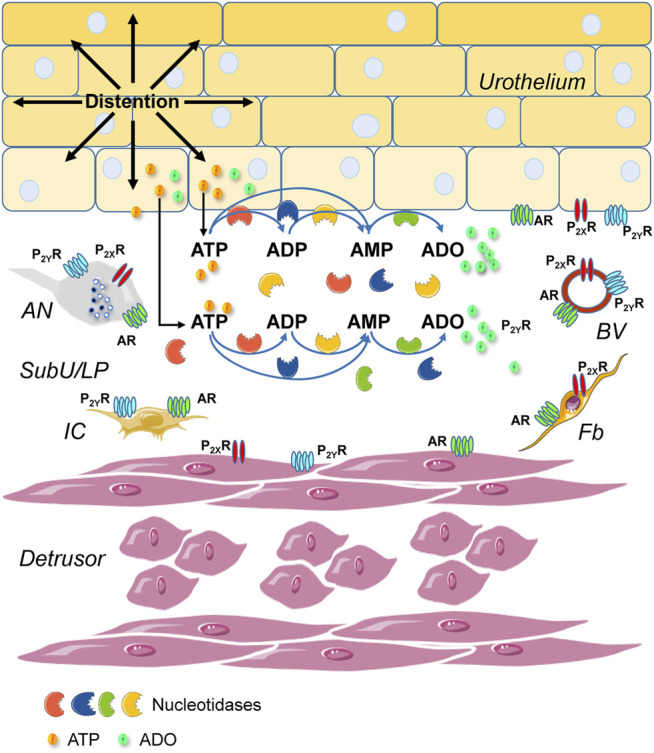
A model depicting mechanisms of purinergic signaling in the lamina propria during bladder filling. Stretch of the bladder wall during filling causes release of ATP from the urothelium into the suburothelium (Birder and Andersson, 2013; Burnstock, 2014; Dalghi et al., 2020). ATP activates P2X (e.g., P2X2/X3) receptors (P2XR) on afferent nerve terminals in urothelium and suburothelium/lamina propria (SubU/LP) and triggers a voiding reflex (Cockayne et al., 2000; Vlaskovska et al., 2001). ATP that is released in the LP is hydrolyzed to ADP, AMP, and adenosine (ADO) by four families of membrane-bound nucleotidases (Zimmermann et al., 2012). Bladder excitability during filling is regulated by excitatory (ATP and ADP) and inhibitory (ADO) purine mediators in the LP that activate specific purinergic receptors. ATP activates ligand-gated P2XR and G protein-coupled P2Y receptors (P2YR), ADP activates P2YR, and ADO activates G-protein coupled adenosine receptors (AR) (Burnstock, 2014). P2XR, P2YR, and AR are ubiquitously expressed in the bladder wall, including in cells in the detrusor, the urothelium, and the LP (Dalghi et al., 2020). Cell types that express purinergic receptors in the LP include afferent neurons (AN), interstitial cells (IC), fibroblasts (Fb), and blood vessels (BV). Nucleotidases have the ability to terminate P2XR or P2YR responses initiated by ATP and to favor the activation of AR or ADP-responding receptors. In addition to the membrane-bound nucleotidases, enzymes that metabolize ATP are released in the LP spontaneously and during distention of the bladder wall during bladder filling. Released enzymes degrade ATP to ADP, AMP, and ADO. The activity of released enzymes is greater in distended LP than in nondistended LP indicating mechanosensitive release of enzymes. Soluble nucleotidases in the LP identify with several membrane-bound nucleotidases and are possibly released in the LP by distention-induced ectodomain shedding (Lichtenthaler et al., 2018). Distention-dependent degradation of ATP by membrane-bound and soluble nucleotidases diminishes the presence of ATP in the LP at the end of bladder filling (Durnin et al., 2019b) to prevent abnormal excitability of the bladder. The proper availability of excitatory and inhibitory purines in the bladder wall is determined by distention-associated purine release and purine metabolism.
