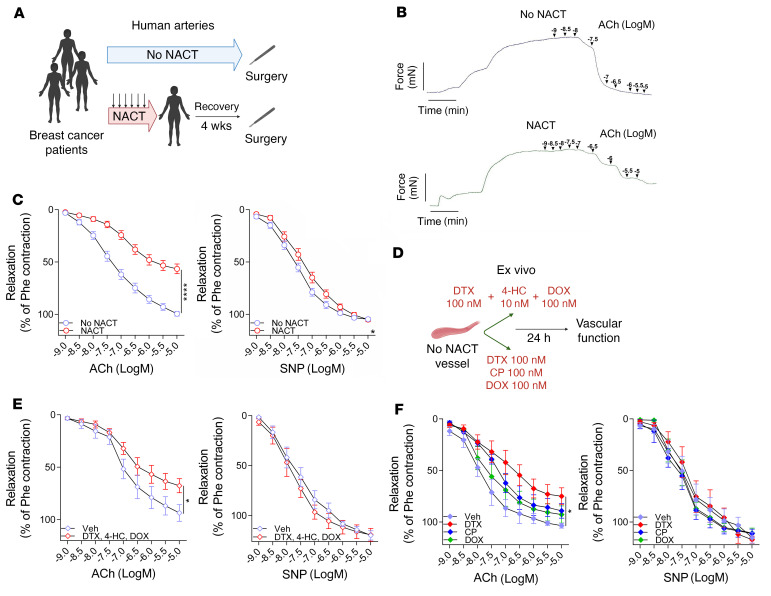
Figure 1. Effects of breast cancer neoadjuvant chemotherapy using docetaxel, cyclophosphamide, and doxorubicin (NACT) on vascular function.
(A) Study design of human blood vessel collection in patients with breast cancer without NACT (No NACT) or after 6 ± 3 courses of NACT prior to surgery. (B) Representative examples of endothelium-dependent vasorelaxations in response to increasing concentrations of ACh (1 nM–10 μM) in arteries from patients with or without NACT. (C) Average endothelium-dependent vasorelaxation responses to ACh (1 nM–10 μM) and endothelium-independent relaxation responses to SNP (1 nM–10 μM) in arteries from patients without NACT (n = 55) and with NACT (n = 40). Two rings were studied per patient, and the values were averaged. Data are expressed as the mean ± SEM. ****P < 0.0001 versus no NACT (C, left); *P < 0.05 versus no NACT (C, right); 2-way, repeated-measures ANOVA. (D) Experimental design of an ex vivo organ culture study of the effects of NACT on vascular function in NACT-naive arteries. (E) Endothelium-dependent (ACh) and endothelium-independent (SNP) vasorelaxations in NACT-naive arteries after a 24-hour organ culture with the combined NACT components docetaxel (100 nM), 4-hydroperoxycyclophosphamide (4-HC) (10 nM), and doxorubicin (100 nM) or vehicle (Veh, solvent) (paired arterial rings for each treatment; n = 7 patients). *P < 0.05 versus vehicle; 2-way, repeated-measures ANOVA. (F) Effects of individual components of NACT on endothelium-dependent (ACh) and endothelium-independent (SNP) vasorelaxations in NACT-naive arteries after a 24-hour organ culture with either docetaxel (100 nM), cyclophosphamide (100 nM), doxorubicin (100 nM), or vehicle (solvent) (paired vessel rings for each treatment; n = 5). *P < 0.05 versus vehicle; 2-way, repeated-measures ANOVA with Tukey’s test. Data in E and F are expressed as the mean ± SEM. DTX, docetaxel; DOX, doxorubicin; CP, cyclophosphamide; Phe, phenylephrine.