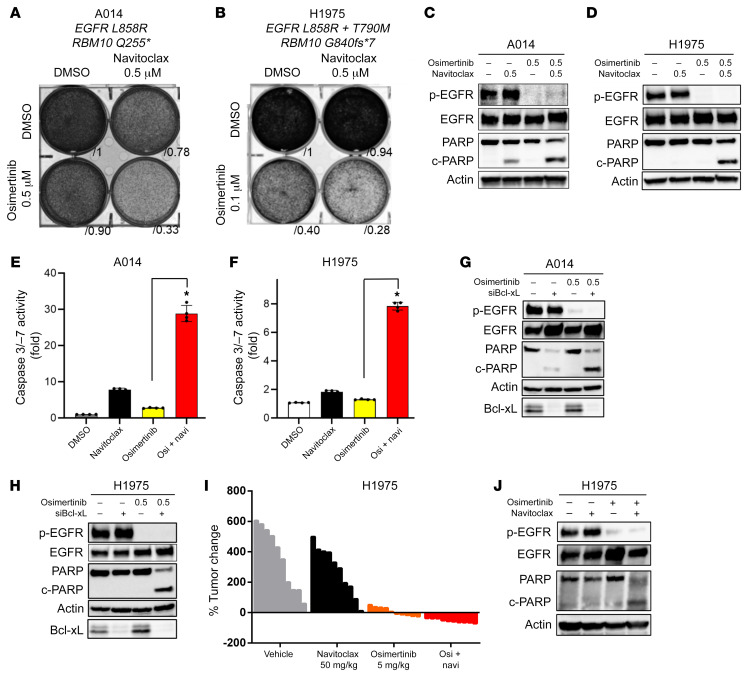
Figure 7. Resistance caused by RBM10 deficiency in EGFR-mutant lung cancer can be overcome with Bcl-xL and EGFR inhibitor combination therapy.
(A–F) RBM10-deficient A014 (EGFR L858R; RBM10 Q255*) and H1975 (EGFR L8585R/T790M; RBM10 G840fs*7) cells were treated with 500 nM navitoclax (ABT-263) alone or in combination with the indicated osimertinib concentrations. (A and B) Crystal violet viability assays were performed, and (C–F) apoptosis was measured according to PARP cleavage and caspase 3/-7 activity. (E and F) Each bar represents the mean ± SEM of the FC after normalization to the DMSO control. (G and H) Western blot analysis of Bcl-xL KD with siRNA in combination with 500 nM osimertinib in A014 and H1975 cells. (I) Mice bearing H1975 subcutaneous xenografts were treated with vehicle, navitoclax (50 mg/kg), osimertinib (5 mg/kg), or their combination (navitoclax plus osimertinib) for 14 days (n = 10 tumors in each group). The percentage of change in tumor volume compared with baseline for individual xenografts is shown. (J) H1975 xenograft tumor explants were treated with vehicle, navitoclax, osimertinib, or their combination (navitoclax plus osimertinib at 50 mg/kg and 5 mg/kg, respectively) for 4 days. One tumor of representative size from each group was harvested 4 hours after treatment on day 5, and the indicated protein levels were determined by Western blot analysis. Data represent 3 independent experiments. *P < 0.05, by 1-way ANOVA.