Abstract
Hyperplastic goblet cells and abundant mucus are significant characteristics of inflammatory colorectal polyps (ICRPs) in miniature dachshunds. In this study, selected mucin gene expressions and goblet cell proportions were evaluated in miniature dachshunds with ICRPs and in healthy dogs. Mucin 2 (MUC2) gene expression was not significantly different among the groups, whereas mucin 5AC (MUC5AC) gene expression was significantly higher in the polypoid lesions than in healthy colonic mucosa. Although the percentage of goblet cells in the upper crypt regions did not significantly differ between the groups, that in the lower crypt regions was significantly decreased in polypoid lesions. In conclusion, increased MUC5AC gene expression and goblet cell proportion changes may be associated with the pathogenesis of ICRPs.
Keywords: canine, goblet cell, inflammatory colorectal polyp, mucin
Inflammatory colorectal polyps (ICRPs) in dogs are characterized by multiple inflammatory polyps localized in the colorectal region, with a high prevalence in miniature dachshunds (MDs) in Japan [11]. The pathogenesis of this disease remains unclear; however, interactions between several factors, including genetic susceptibility and commensal bacterial changes, are reportedly involved [6, 7].
The histopathological features of ICRPs are well-studied, and hyperplastic goblet cells, abundant mucus, and thickened colorectal mucosa are identified as the condition’s distinct characteristics [16]. However whether these changes are associated with the pathogenesis of ICRPs remains unclear. Studies focusing on the characteristics of goblet cells and mucus may therefore prove helpful in understanding the pathogenesis of this disease. Mucins are the main constituent of mucus, and are generally classified into secreted and transmembrane types. Secreted mucins include MUC2, MUC5AC, MUC5B, and MUC6, and their expression patterns differ according to the organ. In the colonic mucosa, mucin 2 (MUC2) is the predominantly secreted mucin [8]. In humans with ulcerative colitis (UC), a major form of inflammatory bowel disease, MUC2 gene expression has been reported to be increased or unchanged [10, 14]. The gene expression of mucin 5AC (MUC5AC), another type of secreted mucin, was increased in the mucosal biopsy samples of patients with UC [14]. In contrast, the number of goblet cells in the large intestine was reduced in patients with UC, especially in the region of the upper crypt [4, 8]. The expression patterns of mucins in ICRPs have not been reported, and the goblet cell proportions in the lesions have not been assessed in detail. Thus, this study aimed to characterize the mucin gene expression patterns and goblet cell proportions in dogs with ICRPs.
Colorectal mucosal samples were obtained by colonoscopy from 14 MDs at the Hokkaido University Veterinary Teaching Hospital between October 2011 and April 2019 for the investigation of tenesmus, hematochezia, and increased frequency of defecation. All dogs were diagnosed with ICRPs based on clinical and histopathological findings from previous studies [11, 16]. Five MDs with ICRPs had treated with prednisolone (0.4–1.6 mg/kg/day), while cyclosporine had been used in 1 MD with ICRPs. The median age of the dogs was 10.3 (range, 6–12) years, with 6 spayed females and 8 males (2 intact and 6 neutered). The median weight was 5.3 (range, 4.4–7.7) kg. Routine hematology, biochemistry, fecal examination, and abdominal ultrasound showed no apparent abnormalities in these dogs, except for increased serum C-reactive protein concentration (>1 mg/dl) in 5 dogs. For enrollment in this study, written informed consent was obtained from the dogs’ owners.
Colonic mucosal specimens were also obtained by colonoscopy from 8 control dogs, including 6 beagles and 2 mongrels, comprising 7 sexually intact females and 1 intact male. The median age of these dogs was 1 (range, 1–9) year and the median weight was 11.3 (range, 9.0–12.8) kg. These control dogs were considered healthy based on their physical examination, routine hematology, biochemistry, fecal examination, and abdominal ultrasound findings.
All dogs underwent colonoscopy under general anesthesia with midazolam, butorphanol, propofol, and isoflurane. For MDs with ICRPs, tissue samples from polypoid lesions were collected. Additionally, macroscopically normal descending colon tsissue samples (non-polypoid lesion), 10–15 cm apart from the polypoid lesions, were obtained from the dogs with ICRPs. Mucosal samples were collected from the descending colons of the control dogs. At least 6 tissue samples were collected from each region, and one or two samples were immediately soaked in RNAlater (Thermo Fisher Scientific, Waltham, MA, USA), kept at 4°C for 24 hr and then transferred to −80°C. The remaining samples were fixed in neutral buffered 10% formalin and embedded in paraffin. These samples were used for histopathology.
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA, USA), with the removal of genomic DNA using RNase-free DNase Set (Qiagen) according to the manufacturer’s instructions. ReverTra ACE qPCR RT Master Mix (Toyobo, Osaka, Japan) was used to synthesize cDNA from 0.5 µg of the total RNA. Quantitative polymerase chain reaction (PCR) was performed using FastStart Essential DNA Green Master (Roche Diagnostics, Mannheim, Germany) and LightCycler Nano (Roche Diagnostics) with a reaction volume of 20 µl. Primer sets used in this study are shown in Table 1. Primers for MUC2 and MUC5AC were designed by a manufacturer service (Takara Bio, Kusatsu, Japan). Three reference genes were selected for accurate quantification based on a previous study [5]. The conditions for amplification were 95°C for 10 min, 45 cycles of PCR (95°C for 10 sec, 60°C for 10 sec, and 72°C for 15 sec) and dissociation (60°C for 20 sec and 95°C for 20 sec). The threshold cycle (CT) values were determined using LightCycler Nano Software version 1.1.0 (Roche Diagnostics). All measurements were performed in duplicate, and the mean value of ΔCT was determined. The relative expression of each target gene was expressed as n-fold differences relative to that of the reference genes.
Table 1. Primer sequences of mucin genes and reference genes used in this study.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Amplicon size | Accession number |
|---|---|---|---|---|
| MUC2 | ATCACACTCATGCCCAATGGA | TTGTTGGAGCAGCCGTCATAA | 116 | XM_022405513 |
| MUC5AC | GAACAGGCTCTGCCAAGCAA | GATGACCTGCTGCTCGTGGTA | 102 | XM_005631734 |
| HMBS | TCACCATCGGAGCCATCT | GTTCCCACCACGCTCTTCT | 112 | XM_546491 |
| RPL32 | TGGTTACAGGAGCAACAAGAAA | CACATCAGCAGCACTTCA | 100 | XM_848016 |
| RPS18 | TGCTCATGTGGTATTGAGGAA | TCTTATACTGGCGTGGATTCTG | 116 | XM_532106 |
MUC, mucin.
Tissue sections of the polypoid lesion from 5 MDs with ICRPs and those of the healthy samples from 5 healthy dogs were used for analyzing the goblet cell proportions in different regions of the crypt. In the MDs with ICRPs, the polypoid lesions taken by rectal pull-through or polypectomy were used. In the healthy dogs, full-thickness colon biopsy samples obtained at the time of euthanasia for reasons unrelated to this study were used. The sections were stained with hematoxylin and eosin (HE) and alcian blue, periodic acid-Schiff (AB-PAS) to detect the mucin containing goblet cells. After staining, the tissue sections were analyzed using Adobe Photoshop CC (Adobe Systems, San Jose, CA, USA). Goblet cells in the upper or lower part of the crypt were counted in the HE-stained samples and normalized by the number of epithelial cells as described previously [4]. Briefly, goblet cells in 3 randomly selected epithelial areas of each part (×400) were counted by counting the vacuoles, and the number of epithelial cells was also counted by counting the number of nuclei in the same crypt.
The experimental procedures in this study were approved by the Animal Care and Use Committee and Laboratory Animal Experimentation Committee, Graduate School of Veterinary Medicine, Hokkaido University (Approval no. 13-0142 and 14-0031).
Statistical analysis was performed using JMP Pro version 14.0 (SAS Institute Inc., Cary, NC, USA). Differences in gene expressions were compared among sample groups using the Kruskal-Wallis test followed by the Steel-Dwass test. Differences in the normalized goblet cell count in each part were compared between groups using the Mann-Whitney U test. P<0.05 was considered significant.
Fourteen MDs were diagnosed with ICRPs; all polypoid lesions exhibited severe inflammatory cell infiltration, including neutrophils, lymphocytes, and macrophages. Two MDs had diffuse small polyps in the colorectal region, 3 had large solitary polyps, and 9 had both large and small polyps. Non-polypoid lesion samples from 14 MDs with ICRPs and colonic mucosal samples from 8 control dogs were judged as histologically normal according to the World Small Animal Veterinary Association guidelines [3].
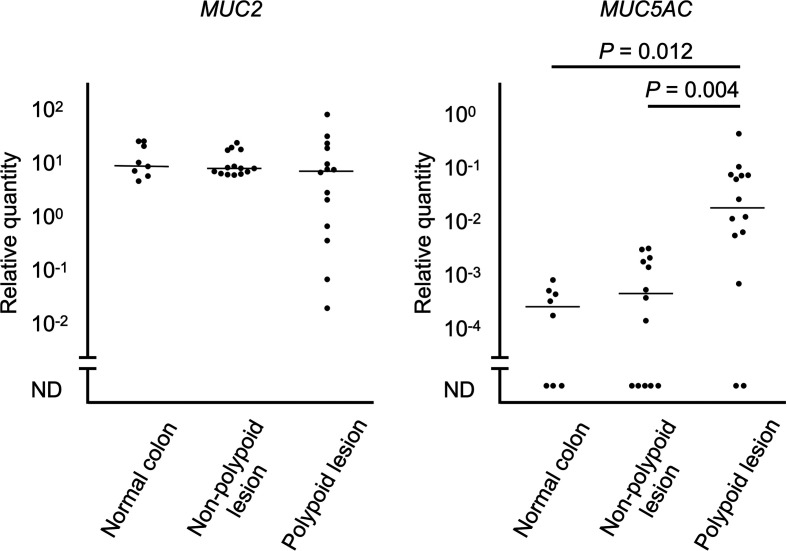
The expressions of each mucin gene are shown in Fig. 1. MUC2 gene expressions were detected in all samples. There was no significant difference in the MUC2 gene expression levels among groups (P=0.43). MUC5AC gene expressions were detected in 5 of 8 (62.5%) normal colonic mucosae, 9 of 14 (64.3%) non-polypoid lesions, and 12 of 14 (85.7%) polypoid lesions. The expression level of MUC5AC in the polypoid lesions was significantly higher than that in the normal colonic mucosa (P=0.012) and non-polypoid lesion (P=0.004).
Fig. 1.
Relative quantity of mucin 2 (MUC2) and mucin 5AC (MUC5AC) mRNA expressions in the polypoid lesion and non-polypoid lesion of inflammatory colorectal polyps (n=14), and normal colonic mucosa of control dogs (n=8). Horizontal lines indicate the median value in each group. ND, not detected.
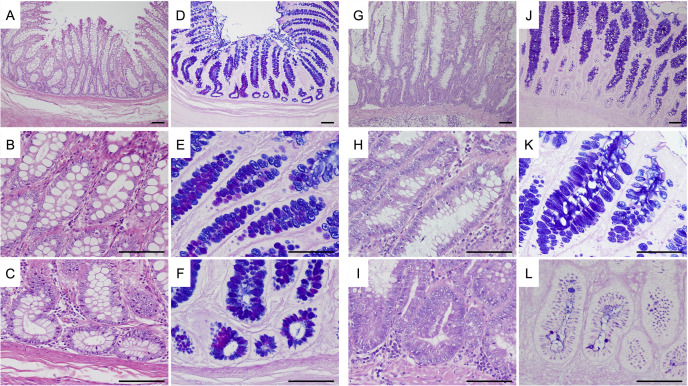
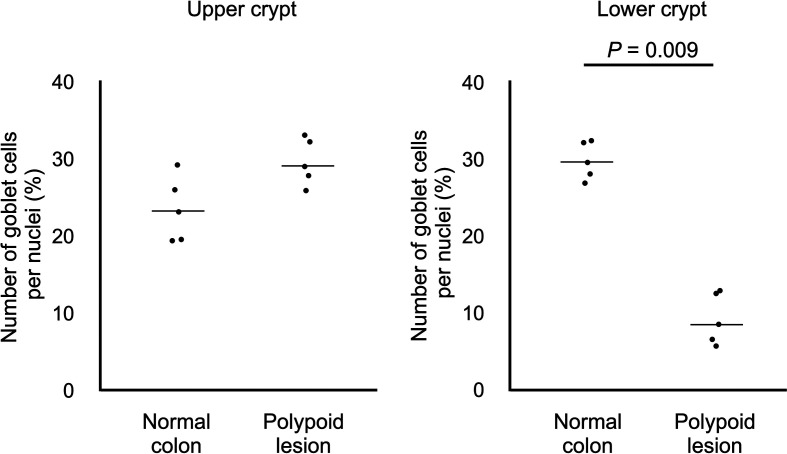
Figure 2 shows the representative images of HE and AB-PAS staining in normal colonic mucosae and epithelial regions of the ICRP polypoid lesions. The percentage of goblet cells in the upper crypt regions was not significantly different between groups (median 23.1% versus 29.0%, P=0.076), while that in the lower crypt regions was significantly decreased in the ICRP polypoid lesions (median 8.5%) compared with that in the normal colonic mucosae (median 29.6%, P=0.009) (Fig. 3).
Fig. 2.
Representative photomicrographs of hematoxylin and eosin (HE), and alcian blue, periodic acid-Schiff (AB-PAS) staining in sections of normal colonic mucosa of healthy dogs, and polypoid lesion of miniature dachshunds with inflammatory colorectal polyps. A–C: low magnification of the crypt (A), upper part of the crypt (B), and lower part of the crypt (C) of normal colonic mucosa (HE). D–F: low magnification of the crypt (D), upper part of the crypt (E), and lower part of the crypt (F) of normal colonic mucosa (AB-PAS). G–I: low magnification of the crypt (G), upper part of the crypt (H), and lower part of the crypt (I) of polypoid lesion (HE). J–L: low magnification of the crypt (J), upper part of the crypt (K), and lower part of the crypt (L) of polypoid lesion (AB-PAS). In each panel, bar=60 µm.
Fig. 3.
The percentage of goblet cells in the upper crypt regions and lower crypt regions in normal colonic mucosa of control dogs (n=5) and polypoid lesions of inflammatory colorectal polyps (n=5). Horizontal lines indicate the median value in each group.
Mucus secreted by goblet cells is important for maintaining the colonic homeostasis by acting as a barrier against mechanical stress and microorganisms [1, 8, 17]. While MUC2 is the main secreted mucin in the colonic mucosa, the gene expression of MUC5AC was also present and increased in the mucosal biopsy samples of patients with UC [14]. In this study, we examined the gene expressions of the secreted mucins, such as MUC2 and MUC5AC, in the polypoid lesions of MDs with ICRPs. Although analyses of other secreted mucins, such as MUC5B, MUC6, and transmembrane types of mucins, are potentially helpful to understand the pathogenesis of this disease, in this study, we focused on MUC2, which is the predominant mucin in the colonic mucosa, and MUC5AC, which has increased expression in human UC [8, 14]. Our results revealed that MUC2 gene expression did not significantly differ among the groups. However, it was expected that the gene expression of MUC2 would be increased in the ICRP lesions because hyperplastic goblet cells with abundant mucus were a histopathological feature of ICRPs [16]. In contrast with MUC2, the gene expression of MUC5AC was higher in the polypoid lesions than in the ICRP non-polypoid and normal colonic mucosa in this study. Although the control dogs in this study were younger than the MDs with ICRP, there was no significant difference between the ICRP non-polypoid and normal colonic mucosa, indicating that age-related changes of gene expression were unlikely. Up-regulated MUC5AC gene expression was considered attributable to high tumor necrosis factor (TNF)-α levels in UC because stimulation of the goblet cell line by TNF-α had reportedly induced MUC5AC secretion in an in vitro model [13]. It is assumed that the increased gene expressions of MUC5AC in the ICRP polypoid lesions were also attributable to the high TNF-α levels, as implicated in previous studies [5, 15]. The MUC5AC mucin is predominantly expressed in the stomach [9], where neutral or PAS positive mucin is generally present [12]. Although we did not objectively evaluate the changes of AB-PAS staining in this study, no remarkable increase in neutral mucin in the polypoid lesions of ICRPs was observed. However, specific cell populations might have expressed PAS positive or MUC5AC mucin. Because we neither evaluated the localization of the MUC2 and MUC5AC genes nor the protein expressions of mucins, further studies on the localization of the genes and their protein expressions in ICRP lesions are needed. Although immunohistochemistry using antibodies is useful to analyze the protein expression and localization of MUC2 and MUC5AC in the ICRPs, commercially available antibodies for use in dogs are not available. Antibodies specific to canine MUC2 and MUC5AC are necessary to evaluate mucin protein expression in ICRPs. In addition, because non-ICRP colitis was not evaluated in this study, whether up-regulation of MUC5AC gene is specific to ICRPs is not clear. Further studies comparing ICRP and non-ICRP colitis are needed.
Although hyperplastic goblet cells and abundant mucus are well-described as the notable characteristics of ICRPs in dogs, the results of this study indicate that the goblet cells in the lower-crypt ICRP polypoid lesions are relatively decreased compared with those of the normal colonic mucosa of healthy dogs. The reason for this difference is unclear; however, several explanations are possible. Mucin secretion from the colonic goblet cells is stimulated by endogenous factors such as acetylcholine and TLR ligands, such as lipopolysaccharide [2]. Treatment using carbachol, an acetylcholine analog, on the crypt led to mucin secretion at the lower but not the upper part of the crypt [2]. Therefore, it is possible that endogenous acetylcholine stimulates the mucin secretion from the lower part of the crypt in ICRP lesions, which seemingly reduces the goblet cell counts. Another hypothesis is that the differentiation and proliferation of goblet cells in the lower part of the crypt is impaired in ICRP polypoid lesions. Colonic goblet cells arise from the stem and progenitor cells in the lower part of the crypt, where several factors are associated with cell differentiation [4]. It is possible that factors involved in goblet cell differentiation and proliferation are impaired in ICRP polypoid lesions. Because we did not evaluate endogenous acetylcholine, goblet cell differentiation factors, or cell proliferation markers, further studies evaluating these factors are needed. For the evaluation of goblet cell proportions, non-polypoid lesions of ICRPs could not be used in this study due to the small tissue size. In future studies, assessment of these factors on both polypoid and non-polypoid lesions is necessary.
In conclusion, this study indicated that the MUC5AC gene, but not MUC2 gene expression, was increased in ICRP polypoid lesions. To the best of our knowledge, this is the first study to evaluate mucin gene expressions in the colorectal mucosa of dogs. Moreover, the goblet cell counts were decreased in the lower part of the crypt in the polypoid lesions. Further studies are needed to clarify the significance of these changes in the pathogenesis of ICRPs.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Yumiko Kagawa for her help with histopathology evaluation, and Dr. Atsushi Kobayashi for his technical assistance.
REFERENCES
- 1.Bankole E., Read E., Curtis M. A., Neves J. F., Garnett J. A.2021. The relationship between mucins and ulcerative colitis: a systematic review. J. Clin. Med. 10: 1935. doi: 10.3390/jcm10091935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birchenough G. M., Nyström E. E., Johansson M. E., Hansson G. C.2016. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 352: 1535–1542. doi: 10.1126/science.aaf7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day M. J., Bilzer T., Mansell J., Wilcock B., Hall E. J., Jergens A., Minami T., Willard M., Washabau R., World Small Animal Veterinary Association Gastrointestinal Standardization Group.2008. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 138Suppl 1: S1–S43. doi: 10.1016/j.jcpa.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 4.Gersemann M., Becker S., Kübler I., Koslowski M., Wang G., Herrlinger K. R., Griger J., Fritz P., Fellermann K., Schwab M., Wehkamp J., Stange E. F.2009. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 77: 84–94. doi: 10.1016/j.diff.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 5.Igarashi H., Ohno K., Maeda S., Kanemoto H., Fukushima K., Uchida K., Tsujimoto H.2014. Expression profiling of pattern recognition receptors and selected cytokines in miniature dachshunds with inflammatory colorectal polyps. Vet. Immunol. Immunopathol. 159: 1–10. doi: 10.1016/j.vetimm.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Igarashi H., Ohno K., Uchida E., Fujiwara-Igarashi A., Kanemoto H., Fukushima K., Uchida K., Tsujimoto H.2015. Polymorphisms of nucleotide-binding oligomerization domain 2 (NOD2) gene in miniature dachshunds with inflammatory colorectal polyps. Vet. Immunol. Immunopathol. 164: 160–169. doi: 10.1016/j.vetimm.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 7.Igarashi H., Ohno K., Horigome A., Fujiwara-Igarashi A., Kanemoto H., Fukushima K., Odamaki T., Tsujimoto H.2016. Fecal dysbiosis in miniature dachshunds with inflammatory colorectal polyps. Res. Vet. Sci. 105: 41–46. doi: 10.1016/j.rvsc.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 8.Johansson M. E.2014. Mucus layers in inflammatory bowel disease. Inflamm. Bowel Dis. 20: 2124–2131. doi: 10.1097/MIB.0000000000000117 [DOI] [PubMed] [Google Scholar]
- 9.Johansson M. E., Sjövall H., Hansson G. C.2013. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10: 352–361. doi: 10.1038/nrgastro.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niv Y.2016. Mucin gene expression in the intestine of ulcerative colitis patients: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 28: 1241–1245. doi: 10.1097/MEG.0000000000000707 [DOI] [PubMed] [Google Scholar]
- 11.Ohmi A., Tsukamoto A., Ohno K., Uchida K., Nishimura R., Fukushima K., Takahashi M., Nakashima K., Fujino Y., Tsujimoto H.2012. A retrospective study of inflammatory colorectal polyps in miniature dachshunds. J. Vet. Med. Sci. 74: 59–64. doi: 10.1292/jvms.11-0352 [DOI] [PubMed] [Google Scholar]
- 12.Sheahan D. G., Jervis H. R.1976. Comparative histochemistry of gastrointestinal mucosubstances. Am. J. Anat. 146: 103–131. doi: 10.1002/aja.1001460202 [DOI] [PubMed] [Google Scholar]
- 13.Smirnova M. G., Birchall J. P., Pearson J. P.2000. TNF-alpha in the regulation of MUC5AC secretion: some aspects of cytokine-induced mucin hypersecretion on the in vitro model. Cytokine 12: 1732–1736. doi: 10.1006/cyto.2000.0763 [DOI] [PubMed] [Google Scholar]
- 14.Taman H., Fenton C. G., Hensel I. V., Anderssen E., Florholmen J., Paulssen R. H.2018. Transcriptomic landscape of treatment-naïve ulcerative colitis. J. Crohn’s Colitis 12: 327–336. doi: 10.1093/ecco-jcc/jjx139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura Y., Ohta H., Torisu S., Yuki M., Yokoyama N., Murakami M., Lim S. Y., Osuga T., Morishita K., Nakamura K., Yamasaki M., Takiguchi M.2013. Markedly increased expression of interleukin-8 in the colorectal mucosa of inflammatory colorectal polyps in miniature dachshunds. Vet. Immunol. Immunopathol. 156: 32–42. doi: 10.1016/j.vetimm.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 16.Uchida E., Chambers J. K., Nakashima K., Saito T., Ohno K., Tsujimoto H., Nakayama H., Uchida K.2016. Pathologic features of colorectal inflammatory polyps in miniature dachshunds. Vet. Pathol. 53: 833–839. doi: 10.1177/0300985815618436 [DOI] [PubMed] [Google Scholar]
- 17.Yao D., Dai W., Dong M., Dai C., Wu S.2021. MUC2 and related bacterial factors: therapeutic targets for ulcerative colitis. EBioMedicine 74: 103751. doi: 10.1016/j.ebiom.2021.103751 [DOI] [PMC free article] [PubMed] [Google Scholar]