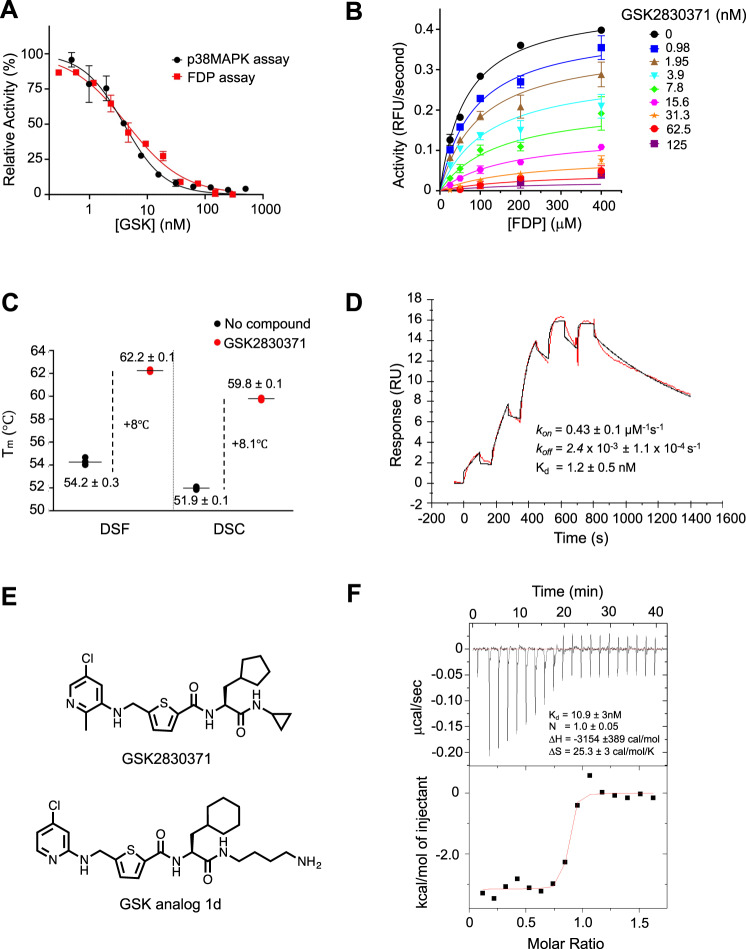
Fig. 3. GSK2830371 functions as a non-competitive inhibitor and binds with high affinity to PPM1D at an allosteric site.
A Activity of GSK2830371 in the FDP (red) and p38 MAPK (black) enzymatic assays. FDP is cleaved by PPM1D in a substrate-independent fashion while p38 MAPK threonine 180 is dephosphorylated in a substrate-specific manner. N = 3 independent samples runs. Data are presented as mean values ± SD. B Mechanism of inhibition studies using non-linear fit of Michaelis–Menten enzyme kinetics where hydrolysis of FDP is measured in relative fluorescence units (RFU). Data suggest noncompetitive mode of action with α ≥ 1. N = 2 independent samples runs. Data are presented as mean values ± SD. C Differential scanning calorimetry (DSC) and fluorimetry (DSF) showing the melting temperature of PPM1D1–420 in the absence (black) and presence (red) of GSK2830371. N = 3 independent sample runs. Data are presented as mean values. D Surface plasmon resonance analysis of the interaction of GSK2830371 with PPM1D1–420. The Kd is calculated from the kon and koff measurements. E Structure of GSK2830371 and the GSK2830371 analog 1d used in the isothermal calorimetry experiments. F The thermodynamic profile of the interaction of the GSK2830371 analog (1d) with PPM1D1–420 as measured by isothermal calorimetry. N = 3 independent sample runs. Source data are provided in the Source Data file.