Abstract
Purpose:
To examine the association between dietary salt intake and glaucoma by antihypertensive use in the Thessaloniki Eye Study (TES) population.
Materials and Methods:
The study population included TES incidence phase participants. Dietary salt intake frequency was assessed by self-report. Outcomes included prevalence of any open angle glaucoma (OAG), primary open angle glaucoma (POAG), and pseudoexfoliation (PEX). Covariates included demographics, cardiovascular disease, migraines, diabetes, steroid use, smoking, history of cataract surgery, central corneal thickness, intraocular pressure, blood pressure, and antihypertensive use. Logistic regression was used to examine associations between frequency of salt intake and glaucoma, controlling for covariates and stratified by antihypertensive use.
Results:
The study included 1,076 participants 80.5±4.4 years old, of whom 518 were female. There were 89/1,076 (8.3%) participants with any OAG, 46/789 (5.8%) with POAG, and 287/1,030 (27.9%) with PEX. In participants with antihypertensive use, frequent versus never salt intake was associated with increased risk of any OAG (adjusted odds ratio [aOR]=2.65, 95% confidence interval [CI]=1.12, 6.28; n=784) and POAG (aOR=3.59, 95% CI=1.16, 11.11; n=578) overall, and additionally in participants with diastolic blood pressure <90mmHg (aOR=2.42, 95% CI=1.00, 5.84; n=735) for OAG. There were no statistically significant adjusted associations between salt intake and PEX, or in participants without antihypertensive use.
Conclusions:
In TES participants assessed for OAG in the prevalence and incidence phases, frequent salt intake may be associated with increased OAG in those who take antihypertensive medication. Further investigation is needed of salt intake and glaucoma in hypertensive individuals.
Keywords: Salt intake, glaucoma, antihypertensive, Thessaloniki, population-based study
Precis
In the Thessaloniki Eye Study incidence phase population, frequent dietary salt intake was potentially associated with increased risk of open-angle glaucoma in antihypertensive users.
Introduction
Glaucoma is a leading cause of permanent vision loss worldwide.1 While reduction of intraocular pressure (IOP) is the mainstay of glaucoma treatment, multiple studies have demonstrated a vascular component in the pathogenesis of glaucoma. Studies have demonstrated associations between both high2,3 and low4–7 blood pressure (BP) and glaucoma risk, as well as associations between low ocular perfusion pressure (OPP) and increased glaucoma risk.8–10 Furthermore, previous studies have suggested that the association between low OPP and glaucoma risk is further influenced by systemic antihypertensive treatment use, possibly related to dysfunctional vascular autoregulation in individuals with hypertension, low ocular perfusion pressure secondary to antihypertensive treatment use, or from greater nocturnal dips to ocular perfusion after antihypertensive treatment.8,11–13
While vascular perfusion has been hypothesized as a contributing factor to the development of glaucoma,14 it is unclear if modifiable risk factors that influence systemic vascular disease, such as sodium and cholesterol intake, also play a role in glaucoma pathogenesis. Sodium is an important regulator of volume homeostasis, and high dietary salt intake is a known risk factor for hypertension and cardiovascular morbidity.15,16 Salt sensitivity refers to the phenomenon where certain individuals have more pronounced blood pressure responses to dietary salt loads compared to other individuals.17,18 Prior literature has suggested that salt-sensitive individuals may be more prone not only to the development of hypertension, but also to suffering its end organ effects such as renal and cardiac failure.19,20 Given the potentially differing associations between OPP and glaucoma in individuals with versus without antihypertensive treatment, there is also a possibility that a salt-sensitive phenomenon exists with glaucoma risk. To this end, the purpose of the present study was to examine the association between dietary salt intake and glaucoma prevalence by antihypertensive status in the Thessaloniki Eye Study (TES) population.
Materials and Methods
The TES is a population-based study of chronic eye diseases based in Thessaloniki, a major urban center in Northern Greece. The study consists of an original prevalence phase with data collection from 2000–2005, and a follow-up incidence phase of surviving cohort members with data collection from 2013–2015.21 Details of study design and data collection have been previously described.21,22 In brief, in the original prevalence phase, a group of 5,000 individuals 60 years and older in the municipality register of the city of Thessaloniki was randomly selected for study recruitment. This group was contacted by phone and mail for willingness to participate in the study. Those who were eligible were invited for an on-site clinic visit at the Thessaloniki Eye Study Center of the Aristotle University of Thessaloniki for an ophthalmic screening examination. Those unable to come for an on-site visit because of illness or major disability were offered a home visit. In the follow-up incidence phase approximately 12 years later, participants from the original prevalence phase were contacted by phone and mail and re-invited for an on-site ophthalmic examination. A home visit examination was again arranged for those who could not come for an on-site visit. The present study included clinic and home visit participants who were examined both in the prevalence and incidence phases of the TES. The Institutional Review Board (IRB) of the Aristotle University Medical School approved the prevalence and incidence phase visits, and the IRB of the University of California, Los Angeles approved the data analyses for the study.
Details of the in-clinic examination for both the TES prevalence and incidence phases have been previously described.21,22 All TES clinic visit participants were interviewed about demographics, systemic conditions (hypertension, diabetes, cardiovascular disease, migraine), ophthalmic conditions (glaucoma, age-related macular degeneration, diabetic retinopathy, cataract, retinal detachment, corneal transplant, uveitis, trauma), and lifestyle factors (smoking, alcohol use, diet, sleep). Participants were instructed to bring systemic and ocular medications to the clinic visit, and medications were verified by study personnel. Blood pressure was measured at the visit with two separate readings at least five minutes apart in the same arm, after the participant had been seated for at least 10 minutes. A standard eye examination was performed on all participants including visual acuity, Goldmann applanation tonometry, gonioscopy, central corneal thickness (CCT), slit lamp biomicroscopy of the anterior segment, dilated fundus exam, and visual field testing. When visual acuity was less than 20/30 with habitual correction, a full refraction was performed and the best corrected visual acuity was recorded. Intraocular pressure (IOP) for each eye was defined as the mean of three IOP readings for that eye. The CCT for each eye was defined as the mean of five CCT measurements for that eye. When the angle was occludable, participants were referred for laser peripheral iridotomy and the dilated fundus exam was completed after iridotomy was performed. Visual field testing was performed using Humphrey automated perimetry. All participants underwent suprathreshold testing, and those with abnormal or unreliable results underwent full threshold testing with 24-2 Swedish Interactive Threshold Algorithm. 76-suprathreshold (STHR) and threshold fields were considered unreliable if the percentage of either fixation losses or false-negative or false-positive errors exceeded 33%.22
The exposure of interest was the level of dietary salt intake as measured by participant report. All participants of the TES incidence phase were asked at their incidence phase visit about level of salt intake. Participants were provided a questionnaire which inquired about the type of salt used to flavor food (ordinary salt, light salt, salt substitute), frequency of salt use at the table (never, rarely, occasionally, often), frequency of salt use while cooking (never, rarely, occasionally, often), and use of salt tablets. Participants who did not cook for themselves were not asked about salt content in their prepared food. The full salt questionnaire is provided in Appendix 1. For this study, participants who reported never using salt at the table and while cooking were considered never salt users, those who reported rare or occasional salt use at the table and while cooking were considered occasional salt users, and those who reported frequent salt use at the table and while cooking were considered frequent salt users. Those who reported combinations of never, occasional, and frequent salt use at the table and while cooking were considered occasional salt users.
The outcomes of interest were the occurrence of any open angle glaucoma (OAG), primary open angle glaucoma (POAG), and pseudoexfoliation syndrome (PEX). Any OAG included participants with either POAG or pseudoexfoliation glaucoma; participants with occludable angles with a history of laser iridotomy who developed subsequent glaucoma were excluded. The PEX outcome included all participants with PEX regardless of whether they had glaucoma. The primary definition of glaucoma was based on the presence of both structural and functional characteristics and has been previously described.11,21,22 Structural damage was defined as thinning or notching of the neuroretinal rim. Functional damage required a confirmed threshold visual field defect. Participants with abnormal visual field results underwent repeat confirmatory testing. A glaucomatous visual field test was confirmed when a defect was present on at least two of the three tests involving the same index on test and retest and occurring in the same location. In cases that had missing data (e.g. participants with poor vision or blindness unable to perform a visual field test), only visual field damage, only disc damage, or high IOP with optic disc findings, a second definition of glaucoma was applied where clinical judgment was strongly in favor of glaucoma even though strict criteria were not met.22 These alternative criteria have been previously described in detail22 and include optic disc criteria only with thinning or notching of the optic disc rim combined with asymmetry of >0.2 of the cup to disc ratio, or IOP asymmetry between the two eyes >4mmHg matching thinning or notching of the optic disc rim combined with asymmetry of >0.2 of the cup to disc ratio. In the latter situation, three glaucoma-trained ophthalmologists were responsible for the ophthalmic examination. At least two of the three examined each patient, and all diagnoses were made by consensus agreement while the assessment of the presence of glaucomatous appearance of the optic disk (thinning or notching) was masked from the three ophthalmologists/ glaucoma specialists. When disagreement between the graders existed, the principal investigator (F.T.) was responsible for the final adjudication of the diagnosis. Pseudoexfoliation was defined as the presence of pseudoexfoliative material at the pupillary margin or on the anterior lens capsule. Slit lamp examination was performed both before and after pupillary dilation to assess for pseudoexfoliation.
Demographic information included age, gender, and marital status. Systemic conditions and history included cardiovascular disease, diabetes, migraines, history of steroid use, and active smoking status. Systemic conditions were assessed based on self-report. Ocular conditions included history of cataract surgery and age-related macular degeneration (ARMD). Examination data included as covariates were systolic blood pressure (SBP), diastolic blood pressure (DBP), IOP, and central corneal thickness. Information on antihypertensive use was collected and used as a variable for stratified analysis to assess the role of antihypertensive use as a potential effect measure modifier.
Descriptive statistics were used to describe baseline characteristics for the study population. Univariable comparisons were made of levels of salt intake in participants with versus without any OAG, POAG, and PEX with chi-squared tests, in the entire study population and stratified by participants with and without antihypertensive use. P-values for interaction were calculated for antihypertensive use by salt intake in participants with any OAG, POAG, and PEX. Logistic regression modeling was used to assess multivariable associations between level of salt intake and any OAG, POAG, and PEX in the entire population and stratified by antihypertensive use, controlling for demographics in a partially adjusted model and all study covariates in a fully adjusted model. In participants with antihypertensive use, additional stratified analyses were performed in participants with diastolic blood pressure (DBP) <90mmHg and ≥90mmHg. The denominator for proportions included all study participants for the any OAG outcome, participants without PEX for the POAG outcome, and participants without POAG for the PEX outcome. All statistical analyses were performed using SAS version 9.4 (Cary, North Carolina).
Results
The study population included 1,076 participants of the TES incidence phase who participated in clinic and/or home visits and the salt intake questionnaire and had a history of no glaucoma or open angle glaucoma (Figure 1). Of these participants, 89/1,076 (8.3%) participants had any OAG; 46/789 (5.8%) participants without PEX had POAG, and 287/1,030 (27.9%) participants without POAG had PEX. Denominators for each type of glaucoma outcome differed due to the exclusion of patients with PEX in the assessment of POAG, and the exclusion of patients with POAG in the assessment of PEX. Of the 89 participants with OAG, 67 were diagnosed based on the presence of structural and functional characteristics, and 22 were diagnosed based on only visual field damage, only disc damage, or high IOP with optic disc findings. There were 1,047/1,076 (97.3%) participants who reported use of ordinary salt, and as such participants were not analyzed separately based on type of salt used. There was 1 participant who reported salt tablet use, and as such the use of salt tablets was not analyzed in this study. There were 784 (72.9%) participants who were on antihypertensive medications. Characteristics of the study population at the time of data collection for the TES incidence phase are outlined in Tables 1 and 2. Compared to participants without any OAG, those with any OAG had thinner CCT (533.8±40.8 versus 545.6±34.9 microns; p=0.003) and higher IOP (18.3±6.7 versus 14.4±2.9 mmHg; p<0.0001). There were no statistically significant differences in any other study covariates in participants with versus without any OAG. There were statistically significant differences in the percentage of participants with migraines, diabetes, and current smoking status between the three levels of salt intake (Table 2), and no statistically significant differences in any other covariates by level of salt intake.
Figure 1.
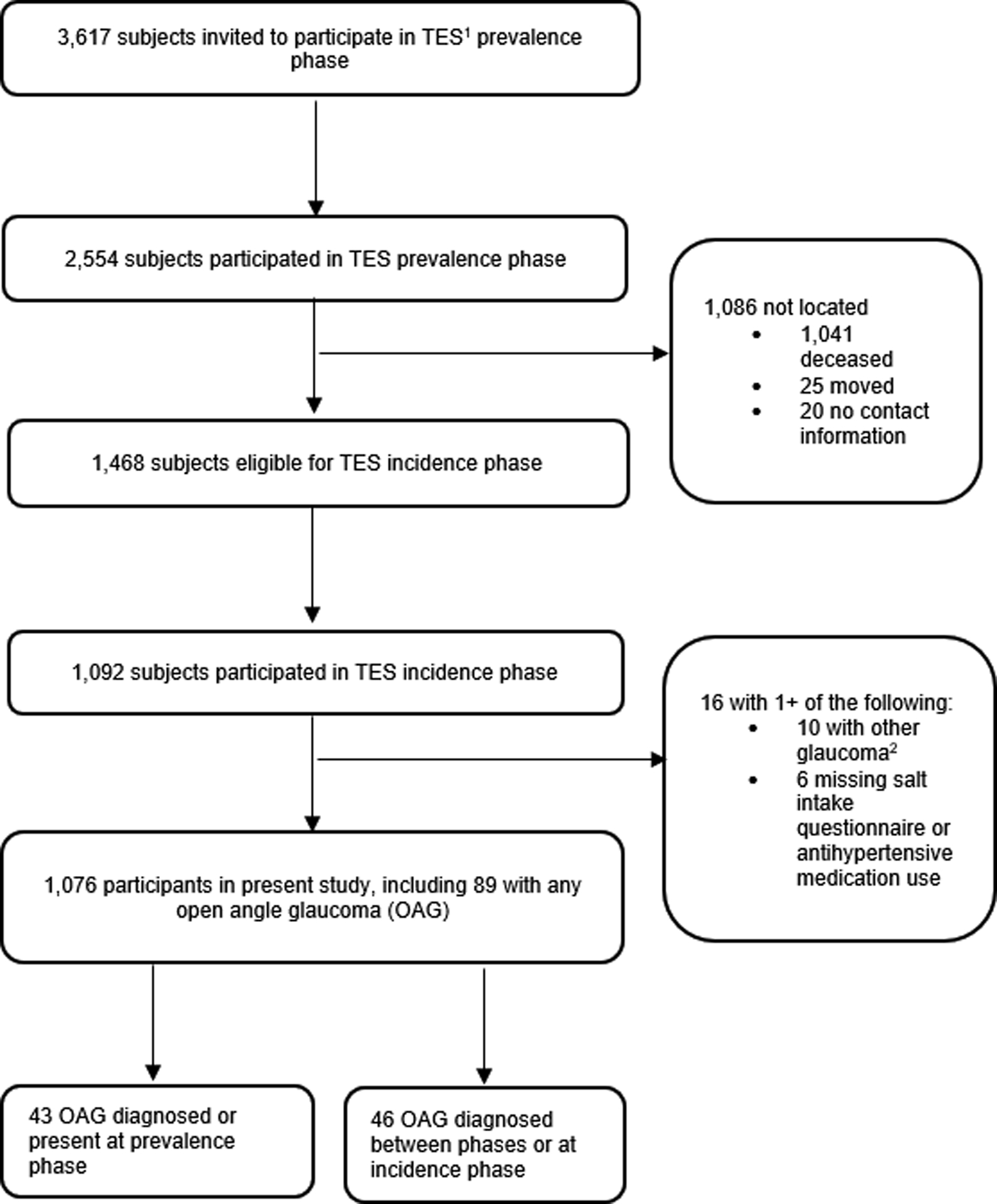
Flowchart of Study Population
1TES=Thessaloniki Eye Study
2Includes types of glaucoma other than primary open angle glaucoma or pseudexfoliation.
Table 1.
Characteristics of Study Population at the Incidence Phase of the Thessaloniki Eye Study by Glaucoma Status (n=1,076)
| Continuous Variables | ||||
|---|---|---|---|---|
| Characteristic | Mean (Standard Deviation) in Entire Population; n=1,076 | Mean (Standard Deviation) in Participants with Any Open Angle Glaucoma; n=89 | Mean (Standard Deviation) in Participants without Open Angle Glaucoma; n=987 | P-Value |
| Age (years) | 80.5 (4.4) | 81.2 (4.2) | 80.5 (4.4) | 0.1 |
| Central corneal thickness (μm) | 544.6 (35.6) | 533.8 (40.8) | 545.6 (34.9) | 0.003 |
| Intraocular pressure (mmHg) | 14.7 (3.5) | 18.3 (6.7) | 14.4 (2.9) | <0.0001 |
| Systolic blood pressure (mmHg) | 130.9 (18.0) | 131.1 (16.1) | 130.9 (18.2) | 0.9 |
| Diastolic blood pressure (mmHg) | 71.2 (11.6) | 72.0 (12.7) | 71.1 (11.5) | 0.5 |
| Categorical Variables | ||||
| Characteristic | Number (%) in Entire Population; n=1,076 | Number (%) in Participants with Any Open Angle Glaucoma; n=89 | Number (%) in Participants without Open Angle Glaucoma; n=987 | P-Value |
| Female | 518 (48.1) | 37 (41.6) | 481 (48.7) | 0.2 |
| Divorced/Separated/Widowed | 411 (38.2) | 31 (34.8) | 380 (38.5) | 0.7 |
| Antihypertensive Use | 784 (72.9) | 64 (71.9) | 720 (73.0) | 0.8 |
| Cardiovascular Disease | 414 (38.6) | 33 (37.1) | 381 (38.7) | 0.8 |
| Migraines | 66 (6.1) | 4 (4.5) | 62 (6.3) | 0.6 |
| Diabetes | 241 (22.4) | 26 (29.2) | 215 (21.8) | 0.1 |
| History of Cataract Surgery | 444 (41.3) | 43 (48.3) | 401 (40.6) | 0.1 |
| Steroid Use | 61 (5.7) | 3 (3.4) | 58 (5.9) | 0.5 |
| Active Smoker | 525 (48.8) | 50 (56.2) | 475 (48.1) | 0.2 |
Table 2.
Characteristics of Study Population at the Incidence Phase of the Thessaloniki Eye Study by Salt Intake Status (n=1,076)
| Continuous Variables | ||||
|---|---|---|---|---|
| Characteristic | Mean (Standard Deviation) in Participants with “Never” Frequency of Salt Use; n=347 | Mean (Standard Deviation) in Participants with “Rare/Occasional” Frequency of Salt Use; n=579 | Mean (Standard Deviation) in Participants with “Often” Frequency of Salt use; n=150 | P-Value |
| Age (years) | 80.9 (4.7) | 80.3 (4.3) | 80.4 (4.3) | 0.1 |
| Central corneal thickness (μm) | 543.2 (36.1) | 544.6 (34.1) | 548.1 (39.7) | 0.4 |
| Intraocular pressure (mmHg) | 14.6 (3.4) | 14.7 (3.5) | 14.9 (4.0) | 0.6 |
| Systolic blood pressure (mmHg) | 132.2 (17.6) | 130.3 (18.8) | 130.3 (15.4) | 0.3 |
| Diastolic blood pressure (mmHg) | 71.6 (11.0) | 71.2 (12.2) | 69.9 (10.6) | 0.3 |
| Categorical Variables | ||||
| Characteristic | N (%) in Participants with “Never” Frequency of Salt Use; n=347 | N (%) in Participants with “Rare/Occasional” Frequency of Salt Use; n=579 | N (%) in Participants with “Often” Frequency of Salt use; n=150 | P-Value |
| Female | 178 (51.3) | 272 (47.0) | 68 (45.3) | 0.3 |
| Divorced/Separated/Widowed | 136 (39.2) | 222 (38.3) | 53 (35.3) | 0.5 |
| Antihypertensive Use | 248 (71.5) | 421 (72.7) | 115 (76.7) | 0.5 |
| Cardiovascular Disease | 121 (35.0) | 232 (40.1) | 61 (40.7) | 0.3 |
| Migraines | 13 (3.8) | 39 (6.7) | 14 (9.3) | 0.04 |
| Diabetes | 65 (18.7) | 131 (22.6) | 45 (30.0) | 0.02 |
| History of Cataract Surgery | 144 (41.5) | 236 (40.8) | 64 (42.7) | 0.9 |
| Steroid Use | 17 (4.9) | 37 (6.4) | 7 (4.7) | 0.6 |
| Active Smoker | 147 (42.4) | 297 (51.3) | 81 (54.0) | 0.01 |
Table 3 summarizes univariable comparisons of the frequency of salt intake in participants with versus without any OAG, POAG, and PEX in the entire population and stratified by antihypertensive status. In the entire study population, there were no statistically significant differences in the frequency of salt intake in participants with versus without OAG, POAG, and PEX. In participants on antihypertensive medication, those with any OAG and POAG had higher proportions who reported frequent salt intake, though none of the comparisons were statistically significant. Conversely, in participants not on antihypertensive medication, those with any OAG and POAG had lower proportions who reported frequent salt intake, though again none of the comparisons were statistically significant. Additionally, the p-value for interaction between antihypertensive medication use and salt intake was significant for any OAG, but not for POAG or PEX.
Table 3.
Frequency of Salt Usage by Antihypertensive Use Status in Participants with and without Glaucoma in the Incidence Phase of the Thessaloniki Eye Study (n=1,076)
| Any Open Angle Glaucoma (OAG) | ||||
|---|---|---|---|---|
| Frequency of Salt Use at Table or While Cooking | Number (%) in Participants with Any OAG; n=89 | Number (%) in Participants without OAG; n=987 | P-Value | P for Interaction, Antihypertensive Use*Salt Intake |
| Often | 16 (18.0) | 134 (13.6) | 0.5 | n/a |
| Often | 15 (23.4) | 100 (13.9) | 0.06 | |
| Often | 1 (4.0) | 34 (12.7) | 0.2 | 0.05 |
| Primary Open Angle Glaucoma (POAG) | ||||
| Frequency of Salt Use at Table or While Cooking | Number (%) in Participants with POAG; n=46 | Number (%) in Participants without POAG or Pseudoexfoliation; n=743 | P-Value | P for Interaction, Antihypertensive Use*Salt Intake |
| Often | 10 (21.7) | 107 (14.4) | 0.4 | n/a |
| Often | 10 (30.3) | 83 (15.2) | 0.09 | |
| Often | 0 (0.0) | 24 (12.1) | 0.2 | 0.5 |
| Pseudoexfoliation Syndrome (PEX) | ||||
| Frequency of Salt Use at Table or While Cooking | Number (%) in Participants with PEX; n=287 | Number (%) in Participants without PEX or POAG; n=743 | P-Value | P for Interaction, Antihypertensive Use*Salt Intake |
| Often | 33 (11.5) | 107 (14.4) | 0.2 | n/a |
| Often | 22 (10.7) | 83 (15.2) | 0.05 | |
| Often | 11 (13.6) | 24 (12.1) | 0.9 | 0.3 |
Table 4 summarizes associations between frequency of salt intake and any OAG, POAG, and PEX, in participants with versus without antihypertensive treatment. In the entire study population, there were no statistically associations between frequency of salt intake and odds of OAG, POAG, or PEX in unadjusted, partially adjusted, or fully adjusted models including adjustment for antihypertensive medication use. In antihypertensive users, compared to no salt intake, frequent salt intake was associated with higher odds of any OAG in all models (odds ratio [OR]=2.51, 95% confidence interval [CI]=1.17, 5.39 unadjusted; OR=2.54, 95% CI=1.18, 5.47 partially adjusted; OR=2.65, 95% CI=1.12, 6.28 fully adjusted). Additionally, in antihypertensive users, frequent compared to no salt intake was associated with higher odds of POAG in all models (OR=2.74, 95% CI=1.04, 7.20 adjusted; OR=2.86, 95% CI=1.08, 7.58 partially adjusted; OR=3.59, 95% CI=1.16, 11.11 fully adjusted). There were no statistically significant associations between any level of salt intake and PEX in participants with antihypertensive treatment. In all levels of adjustment, there were no statistically significant associations between any level of salt intake and any type of glaucoma in participants without antihypertensive treatment. For participants with POAG without antihypertensive treatment, reliable statistical estimates could not be produced for unadjusted or adjusted associations.
Table 4.
Associations between Salt Intake and Glaucoma-Related Outcomes in Participants with and without Antihypertensive Use in the Thessaloniki Eye Study Incidence Phase (n=1,076)
| Entire Population (n=1,076) | |||
|---|---|---|---|
| Level of Overall Salt Intake and Prevalent Glaucoma Outcome | Unadjusted Odds Ratio (95% Confidence Interval); p-value | Partially Adjusted Odds Ratio (95% Confidence Interval); p-valuea | Fully Adjusted Odds Ratio (95% Confidence Interval); p-valueb |
| Often Salt vs. Never | 1.47 (0.77, 2.84); 0.25 | 1.53 (0.78, 2.91); 0.22 | 1.38 (0.66, 2.89); 0.39 |
| Often Salt vs. Never | 1.54 (0.67, 3.54); 0.31 | 1.60 (0.69, 3.71); 0.27 | 1.45 (0.56, 5.76); 0.45 |
| Often Salt vs. Never | 0.90 (0.57, 1.42); 0.64 | 0.93 (0.59, 1.49); 0.78 | 0.86 (0.53, 1.39); 0.53 |
| Participants with Antihypertensive Use (n=709) | |||
| Level of Overall Salt Intake and Prevalent Glaucoma Outcome | Unadjusted Odds Ratio (95% Confidence Interval); p-value | Partially Adjusted Odds Ratio (95% Confidence Interval); p-valuea | Fully Adjusted Odds Ratio (95% Confidence Interval); p-valuec |
| Often Salt vs. Never | 2.51 (1.17, 5.39); 0.02 | 2.54 (1.18, 5.47); 0.02 | 2.65 (1.12, 6.28); 0.03 |
| Often Salt vs. Never | 2.74 (1.04, 7.20); 0.04 | 2.86 (1.08, 7.58); 0.03 | 3.59 (1.16, 11.11); 0.03 |
| Often Salt vs. Never | 0.83 (0.48, 1.45); 0.52 | 0.86 (0.49, 1.50); 0.59 | 0.77 (0.43, 1.37); 0.37 |
| Participants without Antihypertensive Use (n=262) | |||
| Level of Overall Salt Intake and Prevalent Glaucoma Outcome | Unadjusted Odds Ratio (95% Confidence Interval); p-value | Partially Adjusted Odds Ratio (95% Confidence Interval); p-valuea | Fully Adjusted Odds Ratio (95% Confidence Interval); p-valuec |
| Often Salt vs. Never | 0.21 (0.03, 1.70); 0.15 | 0.22 (0.03, 1.82); 0.16 | 0.05 (0.00, 1.26); 0.07 |
| Occasional/Often Salt vs. Never | 0.42 (0.14, 1.30); 0.13 | 0.41 (0.12, 1.38); 0.15 | 0.33 (0.08, 1.36); 0.12 |
| Often Salt vs. Never | 1.10 (0.47, 2.56); 0.82 | 1.14 (0.48, 2.68); 0.77 | 1.05 (0.42, 2.64); 0.92 |
Adjusted for age, gender, and marital status
Adjusted for age, gender, marital status, antihypertensive use, cardiovascular disease, migraine, diabetes, history of cataract surgery, steroid use, active smoking status, systolic blood pressure, diastolic blood pressure, intraocular pressure, and central corneal thickness
Adjusted for age, gender, marital status, cardiovascular disease, migraine, diabetes, history of cataract surgery, steroid use, active smoking status, systolic blood pressure, diastolic blood pressure, intraocular pressure, and central corneal thickness
Table 5 summarizes associations between frequency of salt intake and any OAG, POAG, and PEX in antihypertensive users with DBP <90mmHg and ≥90mmHg. In participants with DBP <90mmHg and antihypertensive use, frequent compared to no salt intake was associated with increased odds of any OAG in all models (OR=2.30, 95% CI=1.06, 5.02 unadjusted; OR=2.34, 95% CI=1.07, 5.12 partially adjusted; OR=2.42, 95% CI=1.00, 5.84 fully adjusted). There were no statistically significant associations between any level of salt intake and POAG or PEX in participants with antihypertensive use and DBP <90mmHg. In participants with antihypertensive use and DBP ≥90mmHg, there were minimal analyses that could be performed due to small sample sizes and cell counts which produced unstable estimates.
Table 5.
Associations between Salt Intake and Glaucoma-Related Outcomes in Participants with Antihypertensive Use and Diastolic Blood Pressure (DBP) <90mmHg and ≥90mmHg Antihypertensive Use in the Thessaloniki Eye Study Incidence Phase (n=1,076)
| Participants with Antihypertensive Use and DBP <90mmHg (n=735) | |||
|---|---|---|---|
| Level of Overall Salt Intake and Prevalent Glaucoma Outcome | Unadjusted Odds Ratio (95% Confidence Interval); p-value | Partially Adjusted Odds Ratio (95% Confidence Interval); p-valuea | Fully Adjusted Odds Ratio (95% Confidence Interval); p-valueb |
| Often Salt vs. Never | 2.30 (1.06, 5.02); 0.04 | 2.34 (1.07, 5.12); 0.03 | 2.42 (1.00, 5.84); 0.05 |
| Often Salt vs. Never | 2.39 (0.89, 6.42); 0.08 | 2.47 (0.91, 6.69); 0.08 | 3.01 (0.95, 9.53); 0.06 |
| Often Salt vs. Never | 0.77 (0.43, 1.37); 0.38 | 0.80 (0.45, 1.44); 0.46 | 0.71 (0.39, 1.29); 0.27 |
| Participants with Antihypertensive Use and DBP ≥90mmHg (n=49) | |||
| Level of Overall Salt Intake and Prevalent Glaucoma Outcome | Unadjusted Odds Ratio (95% Confidence Interval); p-value | Partially Adjusted Odds Ratio (95% Confidence Interval); p-valuea | Fully Adjusted Odds Ratio (95% Confidence Interval); p-valueb |
| Often Salt vs. Never | -- | -- | -- |
| Often Salt vs. Never | -- | -- | -- |
| Often Salt vs. Never | 3.33 (0.32, 34.82); 0.31 | 3.92 (0.35, 43.69); 0.27 | 19.81 (0.73, 538.17); 0.08 |
Adjusted for age, gender, and marital status
Adjusted for age, gender, marital status, cardiovascular disease, migraine, diabetes, history of cataract surgery, steroid use, active smoking status, systolic blood pressure, diastolic blood pressure, intraocular pressure, and central corneal thickness
Discussion
This study examined the association between frequency of dietary salt intake and any OAG, POAG, and PEX in participants of the TES incidence phase by antihypertensive status. While there was no association between frequency of salt intake and prevalence of OAG, POAG, or PEX in the overall study population, a potential association was found between frequent dietary salt intake and increased prevalence of any OAG and POAG in antihypertensive users overall and in those with DBP <90mmHg. No significant associations were found between salt intake and PEX in any participants with or without antihypertensive use. These findings support the role of antihypertensive use as a potential effect measure modifier in the association between salt intake and glaucoma in the TES incidence phase population.
This investigation of dietary salt intake and glaucoma by antihypertensive status was undertaken due to the fact that multiple previous studies within the TES have suggested that antihypertensive use combined with low DBP or low DPP may be associated with increased risk of glaucoma. Specifically, in non-glaucoma subjects from the TES prevalence phase, a subset of participants with DBP <90mmHg after antihypertensive treatment were found to have increased cupping and decreased rim area on Heidelberg Retina Tomograph (HRT) images.23 Furthermore, in the same subset of participants, all classes of antihypertensive medications were found to be associated with larger cup size and higher CDR in subjects with treated SBP <140mmHg or DBP <90mmHg.24 In the entire TES prevalence phase population, low DPP was associated with increased risk of POAG specifically in subjects treated with antihypertensives.11 The authors hypothesized that antihypertensive medication may disrupt vascular autoregulation and decrease the ability for vessels to vasodilate in low perfusion states, which may decrease bloodflow to the optic nerve and increase risk of glaucoma. Additionally, patients on antihypertensive therapy may experience more pronounced nocturnal dips in blood pressure, which may further accelerate the development of glaucoma.
The present study builds on previous TES findings by examining the role of dietary salt intake in participants with and without antihypertensive therapy, in order to examine the association between salt intake and glaucoma with antihypertensive use as a potential effect measure modifier. Increasing blood sodium levels through dietary salt intake or low-dose fludrocortisone has been previously proposed as a mechanism to decrease systemic hypotension to improve optic nerve perfusion in patients with glaucoma.25 While previous studies have not examined associations between dietary salt intake and optic nerve perfusion, one study examined 22 POAG patients with fludrocortisone treatment and found decreased nocturnal dips after the initiation of fludrocortisone treatment.26 However, the association between salt intake and optic nerve perfusion is likely not straightforward, especially in individuals with vascular comorbidities where autoregulation is already compromised. Salt sensitive hypertension refers to the phenomenon where different individuals have different blood pressure sensitivity to salt intake. Individuals with pronounced blood pressure elevation after salt intake likely have impaired renal sodium excretion, leading to chronic systemic hemodynamic changes with altered autoregulation and increased vascular resistance.27 In this scenario, salt loading likely leads to further vascular injury rather than increased perfusion,28 possibly through stimulation of mineralocorticoid receptors. In the present study, participants on antihypertensive treatment with frequent salt intake potentially had higher prevalence of OAG and POAG, while those not on antihypertensive treatment had no association between salt intake and glaucoma prevalence. While a causative mechanism cannot be inferred from these observational findings, our results suggest that increased dietary salt intake may potentially be associated with detrimental effects on glaucoma risk in those with preexisting disruption to vascular autoregulation to which salt intake maybe a contribution. On the other hand, salt intake may be contributing in the initiation and onset of hypertension which then leads to impaired vascular autoregulation. Interestingly, in our previous analyses association of DBP with optic disc structure in non glaucoma participants and of DBP with POAG was found only in those receiving antihypertensive treatment. Our current finding on the association of salt intake with OAG and POAG only in those under antihypertensive treatment reveals a potential role of salt intake in the same pathophysiology pathway. These findings suggest a need to further evaluate the effects of salt intake on perfusion to the optic nerve and any associated glaucomatous damage.
This study has several limitations mainly related to its observational nature. Several study variables, including the salt intake variable and systemic comorbidities, were based on participant self-report, which could lead to the possibility of misclassification bias. Specifically, the salt intake questionnaire does not specifically inquire about sodium or potassium content in foods or salt content of foods for participants who do not cook for themselves, which could lead to further misclassification of salt intake status. While quantitative measures of salt intake such as urinary salt levels may have provided improved exposure information for the present study, these measures were not collected in the TES study and thus not available for analysis. Additionally, participants may have had varying interpretations of occasional and frequent salt use, which could lead to further misclassification of salt intake levels. As in every population-based study the possibility of misclassification of the glaucoma diagnosis could not be excluded. In our analysis we included home visit participants where ancillary testing was not the same. However, we were able to collect relevant data as necessary for adjustments in multivariate models for both clinic and home visit participants. Excluding home visits would make our sample less representative of the general population, especially as home visit participants are likely to present with more comorbidities also affecting the vascular system. In the assessment of antihypertensive medication use, we did not assess the time of day when medications were taken and thus the possibility of excessive nocturnal dips could not be taken into account. While this study utilized information from the TES incidence phase, we still chose to examine prevalence data for all the glaucoma outcomes due to sample size issues with the slow nature of the disease. Thus, the analyses in this study are cross-sectional associations and temporality cannot be inferred. There were some statistical estimates that could not be produced presumably due to small cell sizes for some outcomes and a large number of covariates in the model, and the study sample size may have limited the ability to capture some statistical associations. Additionally, with all observational studies, there is the possibility of uncontrolled confounding and a causative or temporal association cannot be inferred from study findings. Finally, the study was conducted on a single population in Greece and findings may not be generalizable to other populations outside of Thessaloniki.
In summary, this study found that frequent dietary salt intake may be associated with increased prevalence of any OAG and POAG in participants on antihypertensive therapy in the TES incidence phase population. Additional studies are needed to examine pathophysiologic changes to optic nerve vascular supply with dietary salt load.
Supplementary Material
Acknowledgments
Funding/Support:
This research has been co-financed by the European Union (European Social Fund - ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) - Research Funding Program: THALES. Investing in Knowledge Society Through the European Social Fund. This study was supported by the Center for Community Outreach and Policy, Stein Eye Institute, University of California Los Angeles (Los Angeles, California, USA), an unrestricted grant from Research to Prevent Blindness to the UCLA Stein Eye Institute. Victoria Tseng was supported for this work by the Heed Ophthalmic Foundation. Alon Harris is supported by NIH grant (R01EY030851), NSF DMS (1853222/2021192), the New York Eye and Ear (NYEE) Foundation, and in part by a Challenge Grant award from Research to Prevent Blindness (RPB), NY. The sponsor or funding organization had no role in the design or conduct of this research.
Financial Disclosures:
The authors have the following financial disclosures:
Fotis Topouzis: Pfizer (financial support); Novartis (financial support, consultant); Bayer (financial support); Alcon (financial support); Thea (financial support); Omikron (financial support, consultant), Rheon (financial support), Bausch & Lomb (financial support)
Panayiota Founti: Thea (honoraria)
Alon Harris: Alon Harris would like to disclose that he received remuneration from AdOM, Qlaris, Luseed, and Cipla for serving as a consultant, and he serves on the board of AdOM, Qlaris, and Phileas Pharma. Alon Harris holds an ownership interest in AdOM, Luseed, Oxymap, Qlaris, Phileas Pharma, and QuLent. All relationships listed above are pursuant to Icahn School of Medicine’s policy on outside activities.
The remainder of authors have no financial interests to disclose.
Footnotes
Meeting Presentation: This study was presented as a poster at the Association for Research in Vision and Ophthalmology 2019 Annual Meeting in Vancouver, British Columbia.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology 2000;107:1287–1293. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P, Lee AJ, Rochtchina E, Wang JJl. Open-angle glaucoma and systemic hypertension: The Blue Mountains Eye Study. J Glaucoma 2004;13:319–326. [DOI] [PubMed] [Google Scholar]
- 4.Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol 1999;43(1 Suppl):10–16S. [DOI] [PubMed] [Google Scholar]
- 5.Graham SL, Drance SM, Wijsman K, et al. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology 1995;102:61–69. [DOI] [PubMed] [Google Scholar]
- 6.Hayreh SS, Zimmerman MB, Podhajsky P, et al. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol 1994;117:603–624. [DOI] [PubMed] [Google Scholar]
- 7.Collignon N, Dewe W, Guillaume S, et al. Ambulatory blood pressure monitoring in glaucoma patients. The nocturnal systolic dip and its relationship with disease progression. Int Ophthalmol 1998;22:19–25. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Wong TY, Mitchell P, et al. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: The Singapore Malay Eye Study. Invest Ophthalmol Vis Sci 2010;51:3399–3404. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Wu SY, Nemesure B, et al. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol 2002;120:954–959. [DOI] [PubMed] [Google Scholar]
- 10.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology 2007;114:1965–1972. [DOI] [PubMed] [Google Scholar]
- 11.Topouzis F, Wilson MR, Harris A, et al. Association of open-angle glaucoma with perfusion pressure status in the Thessaloniki Eye Study. Am J Ophthalmol 2013;155:843–851. [DOI] [PubMed] [Google Scholar]
- 12.Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol 1995;113:216–221. [DOI] [PubMed] [Google Scholar]
- 13.Memarzadeh F, Ying-Lai M, Chung J, et al. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci 2010;516:2872–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinreb RN and Harris A World Glaucoma Association Consensus Series – 6. Ocular blood flow in glaucoma. Kugler Publications, Amsterdam, the Netherlands. 2009. [Google Scholar]
- 15.Strazzullo P, D’Elia L, Kandala NB, et al. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahl LK, Love RA. Evidence for relationship between sodium (chloride) intake and human essential hypertension. Arch Intern Med 1954;94:525–531. [DOI] [PubMed] [Google Scholar]
- 17.Choi HY, Park HC, Ha SK. Salt sensitivity and hypertension: a paradigm shift from kidney malfunction to vascular endothelial dysfunction. Electrolyte Blood Press 2015;13:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberger MH, Miller JZ, Luft FC, et al. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 1986;8:II127–134. [DOI] [PubMed] [Google Scholar]
- 19.Campese VM. Salt sensitivity in hypertension: renal and cardiovascular implications. Hypertension 1994;23:531–550. [DOI] [PubMed] [Google Scholar]
- 20.Siani A, Guglielmucci F, Farinaro E, et al. Increasing evidence for the role of salt and salt-sensitivity in hypertension. Nutr Met Cardiovasc Dis 2000;10:93–100. [PubMed] [Google Scholar]
- 21.Topouzis F, Founti P, Yu F, et al. Twelve-year incidence and baseline risk factors for pseudoexfoliation: The Thessaloniki Eye Study (An American Ophthalmological Society Thesis). Am J Ophthalmol 2019;206:192–214. [DOI] [PubMed] [Google Scholar]
- 22.Topouzis F, Wilson MR, Harris A, et al. Prevalence of open angle glaucoma in Greece: the Thessaloniki Eye Study. Am J Ophthalmol 2007;144:511–519. [DOI] [PubMed] [Google Scholar]
- 23.Topouzis F, Coleman AL, Harris A, et al. Association of blood pressure status with the optic disk structure in non-glaucoma subjects: The Thessaloniki Eye Study. Am J Ophthalmol 2006;142:60–67. [DOI] [PubMed] [Google Scholar]
- 24.Harris A, Topouzis F, Wilson MR, et al. Association of the optic disc structure with the use of antihypertensive medications: The Thessaloniki Eye Study. J Glaucoma 2013;22:526–531. [DOI] [PubMed] [Google Scholar]
- 25.Mozaffarieh M, Flammer J. Is there more to glaucoma treatment than lowering IOP? Surv Ophthalmol 2007;52 suppl 2:S174–179. [DOI] [PubMed] [Google Scholar]
- 26.Gugleta K, Orgul S, Stumpfig D, et al. Fludrocortisone in the treatment of systemic hypotension in primary open-angle glaucoma patients. Int Ophthalmol 1999;23:25–30. [DOI] [PubMed] [Google Scholar]
- 27.Ando K, Fujita T. Pathophysiology of salt sensitivity hypertension. Ann Med 2012;44 suppl 1:S119–126. [DOI] [PubMed] [Google Scholar]
- 28.Fujita T. Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension 2010;55:813–818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


